Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: the IASO trial
- PMID: 30772849
- PMCID: PMC6398753
- DOI: 10.1136/bmjopen-2018-023765
Trial summary and protocol for a phase II randomised placebo-controlled double-blinded trial of Interleukin 1 blockade in Acute Severe Colitis: the IASO trial
Abstract
Introduction:
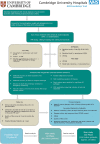
Acute severe ulcerative colitis (ASUC) is a severe manifestation of ulcerative colitis (UC) that warrants hospitalisation. Despite significant advances in therapeutic options for UC and in the medical management of steroid-refractory ASUC, the initial treatment paradigm has not changed since 1955 and is based on the use of intravenous corticosteroids. This treatment is successful in approximately 50% of patients but failure of this and subsequent medical therapy still occurs, with colectomy rates of up to 40% reported. The
Methods and analysis: IASO is a phase II, multicentre, two-arm (parallel group), randomised (1:1), placebo-controlled, double-blinded trial of short-duration anakinra in ASUC. Its primary outcome will be the incidence of medical (eg, infliximab/ciclosporin) or surgical rescue therapy (colectomy) within 10 days following the commencement of intravenous corticosteroid therapy. Secondary outcomes will include disease activity, time to clinical response, time to rescue therapy, colectomy incidence by day 98 post intravenous corticosteroids and safety. The trial aims to recruit 214 patients across 20 sites in the UK.
Ethics and dissemination: The trial has received approval from the Cambridge Central Research Ethics Committee (Ref: 17/EE/0347), the Health Research Authority (Ref: 201505) and Clinical Trials Authorisation from the Medicines and Healthcare products Regulatory Agency. We plan to present trial findings at scientific conferences and publish in high-impact peer-reviewed journals.
Trial registration number: ISRCTN43717130; EudraCT 2017-001389-10.
Keywords: gastroenterology; immunology; inflammatory bowel disease.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Team NP. Table 36 third patient report of the national emergency laparotomy audit. 2017. http://www.nela.org.uk/reports
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
