Thymic size is increased by infancy, but not pregnancy, nutritional supplementation in rural Gambian children: a randomized clinical trial
- PMID: 30773140
- PMCID: PMC6378709
- DOI: 10.1186/s12916-019-1264-2
Thymic size is increased by infancy, but not pregnancy, nutritional supplementation in rural Gambian children: a randomized clinical trial
Abstract
Background: Thymic size in early infancy predicts subsequent survival in low-income settings. The human thymus develops from early gestation, is most active in early life and is highly sensitive to malnutrition. Our objective was to test whether thymic size in infancy could be increased by maternal and/or infant nutritional supplementation.
Methods: The Early Nutrition and Immune Development (ENID) Trial was a randomized 2 × 2 × 2 factorial, partially blinded trial of nutritional supplementation conducted in rural Gambia, West Africa. Pregnant women (N = 875) were randomized to four intervention groups (iron-folate (standard care), multiple micronutrients, protein energy or protein energy + multiple micronutrients at 'booking' (mean gestational age at enrolment = 13.6 weeks, range 8-20 weeks) until delivery. The iron-folate and multiple micronutrient arms were administered in tablet form and the protein energy arms as a lipid-based nutritional supplement. All intervention arms contained 60 mg iron and 400 μg folic acid per daily dose. From 24 to 52 weeks of age, infants from all groups were randomized to receive a daily lipid-based nutritional supplement, with or without additional micronutrients. Thymic size was assessed by ultrasonography at 1, 8, 24 and 52 weeks of infant age, and a volume-related thymic index calculated. Detailed data on infant growth, feeding status and morbidity were collected.
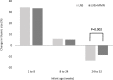
Results: A total of 724 (82.7%) mother-infant pairs completed the trial to infant age 52 weeks. Thymic size in infancy was not significantly associated with maternal supplement group at any post-natal time point. Infants who received the daily LNS with additional micronutrients had a significantly larger thymic index at 52 weeks of age (equivalent to an 8.0% increase in thymic index [95% CI 2.89, 13.4], P = 0.002). No interaction was observed between maternal and infant supplement groups.
Conclusions: A micronutrient-fortified lipid-based supplement given in the latter half of infancy increased thymic size, a key mediator of immune function. Improving the micronutrient status of infants from populations with marginal micronutrient status may improve immune development and survival.
Trial registration: ISRCTN registry (controlled-trials.com) Identifier: ISRCTN49285450.
Keywords: DOHaD; Gambia; Infants; Nutritional supplementation; Pregnancy; Thymus.
Conflict of interest statement
Ethics approval and consent to participate
The trial was approved by the MRC Scientific Coordinating Committee and the joint Gambia Government/MRC Ethics Committee (SCC1126v2). Participating women provided signed consent following a verbal and written description of the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. - DOI - PubMed
-
- Smith ER, Shankar AH, Wu LS, Aboud S, Adu-Afarwuah S, Ali H, Agustina R, Arifeen S, Ashorn P, Bhutta ZA, et al. Modifiers of the effect of maternal multiple micronutrient supplementation on stillbirth, birth outcomes, and infant mortality: a meta-analysis of individual patient data from 17 randomised trials in low-income and middle-income countries. Lancet Glob Health. 2017;5(11):e1090–e1100. doi: 10.1016/S2214-109X(17)30371-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

