Plasma Androgen Receptor and Docetaxel for Metastatic Castration-resistant Prostate Cancer
- PMID: 30773204
- PMCID: PMC6377278
- DOI: 10.1016/j.eururo.2018.09.049
Plasma Androgen Receptor and Docetaxel for Metastatic Castration-resistant Prostate Cancer
Abstract
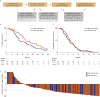
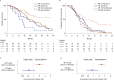
Plasma androgen receptor (AR) gain identifies metastatic castration-resistant prostate cancer (mCRPC) patients with worse outcome on abiraterone/enzalutamide, but its relevance in the context of taxane chemotherapy is unknown. We aimed to evaluate whether docetaxel is active regardless of plasma AR and to perform an exploratory analysis to compare docetaxel with abiraterone/enzalutamide. This multi-institutional study was a pooled analysis of AR status, determined by droplet digital polymerase chain reaction, on pretreatment plasma samples. We evaluated associations between plasma AR and overall/progression-free survival (OS/PFS) and prostate-specific antigen (PSA) response rate in 163 docetaxel-treated patients. OS was significantly shorter in case of AR gain (hazard ratio [HR]=1.61, 95% confidence interval [CI]=1.08-2.39, p=0.018), but not PFS (HR=1.04, 95% CI 0.74-1.46, p=0.8) or PSA response (odds ratio=1.14, 95% CI=0.65-1.99, p=0.7). We investigated the interaction between plasma AR and treatment type after incorporating updated data from our prior study of 73 chemotherapy-naïve, abiraterone/enzalutamide-treated patients, with data from 115 first-line docetaxel patients. In an exploratory analysis of mCRPC patients receiving first-line therapies, a significant interaction was observed between plasma AR and docetaxel versus abiraterone/enzalutamide for OS (HR=0.16, 95% CI=0.06-0.46, p<0.001) and PFS (HR=0.31, 95% CI=0.12-0.80, p=0.02). Specifically, we reported a significant difference for OS favoring abiraterone/enzalutamide for AR-normal patients (HR=1.93, 95% CI=1.19-3.12, p=0.008) and a suggestion favoring docetaxel for AR-gained patients (HR=0.53, 95% CI=0.24-1.16, p=0.11). These data suggest that AR-normal patients should receive abiraterone/enzalutamide and AR-gained could benefit from docetaxel. This treatment selection merits prospective evaluation in a randomized trial. PATIENT SUMMARY: We investigated whether plasma androgen receptor (AR) predicted outcome in metastatic castration-resistant prostate cancer (mCRPC) patients treated with docetaxel, and we performed an exploratory analysis in patients treated with docetaxel or AR-directed drugs as first-line mCRPC therapy. We showed that plasma AR normal favored hormonal treatment, whilst plasma AR-gained patients may have had a longer response to docetaxel, suggesting that plasma AR status could be a useful treatment selection biomarker.
Keywords: Androgen receptor; Androgen receptor–directed therapies; Biomarker; Castration-resistant prostate cancer; Docetaxel; Plasma DNA.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Androgen receptor gene status in plasma DNA associates with worse outcome on enzalutamide or abiraterone for castration-resistant prostate cancer: a multi-institution correlative biomarker study.Ann Oncol. 2017 Jul 1;28(7):1508-1516. doi: 10.1093/annonc/mdx155. Ann Oncol. 2017. PMID: 28472366 Free PMC article. Clinical Trial.
-
Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer.JAMA Oncol. 2015 Aug;1(5):582-91. doi: 10.1001/jamaoncol.2015.1341. JAMA Oncol. 2015. PMID: 26181238 Free PMC article.
-
AR-V7 in Peripheral Whole Blood of Patients with Castration-resistant Prostate Cancer: Association with Treatment-specific Outcome Under Abiraterone and Enzalutamide.Eur Urol. 2017 Nov;72(5):828-834. doi: 10.1016/j.eururo.2017.07.024. Epub 2017 Aug 14. Eur Urol. 2017. PMID: 28818355
-
Cabazitaxel versus abiraterone or enzalutamide for metastatic castration-resistant prostate cancer following docetaxel failure: a systematic review and meta-analysis.Clin Transl Oncol. 2025 Aug;27(8):3465-3476. doi: 10.1007/s12094-025-03851-y. Epub 2025 Feb 22. Clin Transl Oncol. 2025. PMID: 39987332
-
Efficacy and safety of second-line agents for treatment of metastatic castration-resistant prostate cancer progressing after docetaxel. A systematic review and meta-analysis.Arch Ital Urol Androl. 2015 Jul 7;87(2):121-9. doi: 10.4081/aiua.2015.2.121. Arch Ital Urol Androl. 2015. PMID: 26150028
Cited by
-
Identification of Neoantigens and Construction of Immune Subtypes in Prostate Adenocarcinoma.Front Genet. 2022 Apr 25;13:886983. doi: 10.3389/fgene.2022.886983. eCollection 2022. Front Genet. 2022. PMID: 35547260 Free PMC article.
-
Impact of Somatic DNA Repair Mutations on the Clinical Outcomes of Bone Metastases from Castration-Resistant Prostate Cancer.Int J Mol Sci. 2023 Aug 4;24(15):12436. doi: 10.3390/ijms241512436. Int J Mol Sci. 2023. PMID: 37569810 Free PMC article.
-
Androgen receptor gain in circulating free DNA and splicing variant 7 in exosomes predict clinical outcome in CRPC patients treated with abiraterone and enzalutamide.Prostate Cancer Prostatic Dis. 2021 Jun;24(2):524-531. doi: 10.1038/s41391-020-00309-w. Epub 2021 Jan 26. Prostate Cancer Prostatic Dis. 2021. PMID: 33500577 Free PMC article.
-
Validation of a Combined Prognostic Score Using Plasma Tumor DNA and Clinical Features in Metastatic Castration-Resistant Prostate Cancer Patients Treated with Taxanes.Onco Targets Ther. 2025 May 23;18:667-677. doi: 10.2147/OTT.S509285. eCollection 2025. Onco Targets Ther. 2025. PMID: 40438313 Free PMC article.
-
Evaluation of Commercial Circulating Tumor DNA Test in Metastatic Prostate Cancer.JCO Precis Oncol. 2019 Jun 12;3:PO.19.00014. doi: 10.1200/PO.19.00014. eCollection 2019. JCO Precis Oncol. 2019. PMID: 32914020 Free PMC article.
References
-
- Annala M., Vandekerkhove G., Khalaf D. Circulating tumor DNA genomics correlate with resistance to abiraterone and enzalutamide in prostate cancer. Cancer Discov. 2018;8:444–457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

