Monogenic causes of chronic kidney disease in adults
- PMID: 30773290
- PMCID: PMC6431580
- DOI: 10.1016/j.kint.2018.10.031
Monogenic causes of chronic kidney disease in adults
Abstract
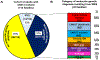
Approximately 500 monogenic causes of chronic kidney disease (CKD) have been identified, mainly in pediatric populations. The frequency of monogenic causes among adults with CKD has been less extensively studied. To determine the likelihood of detecting monogenic causes of CKD in adults presenting to nephrology services in Ireland, we conducted whole exome sequencing (WES) in a multi-centre cohort of 114 families including 138 affected individuals with CKD. Affected adults were recruited from 78 families with a positive family history, 16 families with extra-renal features, and 20 families with neither a family history nor extra-renal features. We detected a pathogenic mutation in a known CKD gene in 42 of 114 families (37%). A monogenic cause was identified in 36% of affected families with a positive family history of CKD, 69% of those with extra-renal features, and only 15% of those without a family history or extra-renal features. There was no difference in the rate of genetic diagnosis in individuals with childhood versus adult onset CKD. Among the 42 families in whom a monogenic cause was identified, WES confirmed the clinical diagnosis in 17 (40%), corrected the clinical diagnosis in 9 (22%), and established a diagnosis for the first time in 16 families referred with CKD of unknown etiology (38%). In this multi-centre study of adults with CKD, a molecular genetic diagnosis was established in over one-third of families. In the evolving era of precision medicine, WES may be an important tool to identify the cause of CKD in adults.
Keywords: chronic kidney disease; genetic kidney disease; whole exome sequencing.
Copyright © 2019 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
No conflict of interests
Figures


Comment in
-
Expanding opportunities and emerging challenges: broadening the scope of genetic testing in nephrology.Kidney Int. 2019 Apr;95(4):743-746. doi: 10.1016/j.kint.2018.12.032. Kidney Int. 2019. PMID: 30904063 Free PMC article.
References
-
- Go AS, Chertow GM, Fan D, et al. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events, and Hospitalization. New England Journal of Medicine 2009; 351: 1296–1305. - PubMed
-
- Gansevoort RT, Correa-Rotter R, Hemmelgarn BR, et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. The Lancet 2013; 382: 339–352. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

