Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering
- PMID: 30773293
- PMCID: PMC6400828
- DOI: 10.1016/j.bpj.2019.01.017
Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering
Abstract
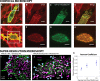
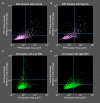
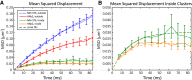
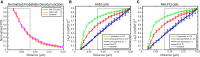
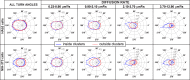
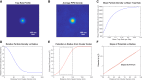
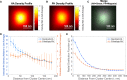
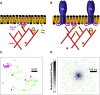
The lipid phosphatidylinositol 4,5-bisphosphate (PIP2) forms nanoscopic clusters in cell plasma membranes; however, the processes determining PIP2 mobility and thus its spatial patterns are not fully understood. Using super-resolution imaging of living cells, we find that PIP2 is tightly colocalized with and modulated by overexpression of the influenza viral protein hemagglutinin (HA). Within and near clusters, HA and PIP2 follow a similar spatial dependence, which can be described by an HA-dependent potential gradient; PIP2 molecules move as if they are attracted to the center of clusters by a radial force of 0.079 ± 0.002 pN in HAb2 cells. The measured clustering and dynamics of PIP2 are inconsistent with the unmodified forms of the raft, tether, and fence models. Rather, we found that the spatial PIP2 distributions and how they change in time are explained via a novel, to our knowledge, dynamic mechanism: a radial gradient of PIP2 binding sites that are themselves mobile. This model may be useful for understanding other biological membrane domains whose distributions display gradients in density while maintaining their mobility.
Copyright © 2019 Biophysical Society. All rights reserved.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

