High-Copy-Number Plasmid Segregation-Single-Molecule Dynamics in Single Cells
- PMID: 30773297
- PMCID: PMC6400962
- DOI: 10.1016/j.bpj.2019.01.019
High-Copy-Number Plasmid Segregation-Single-Molecule Dynamics in Single Cells
Abstract
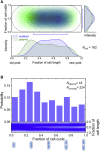
Bacterial high-copy-number (hcn) plasmids provide an excellent model to study the underlying physical mechanisms of DNA segment segregation in an intracellular context. Using two-color fluorescent repressor-operator systems and a synthetic repressible replication origin, we tracked the motion and segregation of single hcn plasmid molecules in individual cells. The plasmid diffusion dynamics revealed between-plasmid temporal associations (clustering) as well as entropic and elastic recoiling forces in the confined intracellular spaces outside of nucleoids. These two effects could be effectively used in models to predict the heterogeneity of segregation. Additionally, the motile behaviors of hcn plasmids provide quantitative estimates of entropic exclusion strength and dynamic associations between DNA segments. Overall, this study utilizes a, to our knowledge, novel approach to predict the polymer dynamics of DNA segments in spatially confined, crowded cellular compartments as well as during bacterial chromosome segregation.
Copyright © 2019 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Murray A.W. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992;359:599–604. - PubMed
-
- Ruchaud S., Carmena M., Earnshaw W.C. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 2007;8:798–812. - PubMed
-
- Reyes-Lamothe R., Nicolas E., Sherratt D.J. Chromosome replication and segregation in bacteria. Annu. Rev. Genet. 2012;46:121–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

