Serine Metabolism Supports Macrophage IL-1β Production
- PMID: 30773464
- PMCID: PMC6447453
- DOI: 10.1016/j.cmet.2019.01.014
Serine Metabolism Supports Macrophage IL-1β Production
Abstract
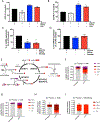
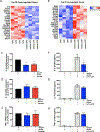
Serine is a substrate for nucleotide, NADPH, and glutathione (GSH) synthesis. Previous studies in cancer cells and lymphocytes have shown that serine-dependent one-carbon units are necessary for nucleotide production to support proliferation. Presently, it is unknown whether serine metabolism impacts the function of non-proliferative cells, such as inflammatory macrophages. We find that in macrophages, serine is required for optimal lipopolysaccharide (LPS) induction of IL-1β mRNA expression, but not inflammasome activation. The mechanism involves a requirement for glycine, which is made from serine, to support macrophage GSH synthesis. Cell-permeable GSH, but not the one-carbon donor formate, rescues IL-1β mRNA expression. Pharmacological inhibition of de novo serine synthesis in vivo decreased LPS induction of IL-1β levels and improved survival in an LPS-driven model of sepsis in mice. Our study reveals that serine metabolism is necessary for GSH synthesis to support IL-1β cytokine production.
Keywords: IL-1beta; LPS response; glutathione; immunometabolism; inflammation; macrophage; one-carbon metabolism; sepsis; serine metabolism.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures


References
-
- Baardman J, Verberk S, Prange K, Weeghel M, Velden S, Ryan D, Wüst R, Neele A, Speijer D, Denis S, et al. (2018). A Defective Pentose Phosphate Pathway Reduces Inflammatory Macrophage Responses during Hypercholesterolemia. Cell Reports 25, 2044–2052.e5. - PubMed
-
- Broz P, and Dixit VM. (2016). Inflammasomes: mechanism of assembly, regulation and signalling. Nature Review Immunology, 16:407–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

