Cancer Metabolism Drives a Stromal Regenerative Response
- PMID: 30773467
- PMCID: PMC6692899
- DOI: 10.1016/j.cmet.2019.01.015
Cancer Metabolism Drives a Stromal Regenerative Response
Abstract
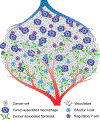
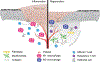
The metabolic reprogramming associated with malignant transformation has led to a growing appreciation of the nutrients required to support anabolic cell growth. Less well studied is how cancer cells satisfy those demands in vivo, where they are dispersed within a complex microenvironment. Tumor-associated stromal components can support tumor growth by providing nutrients that supplement those provided by the local vasculature. These non-malignant stromal cells are phenotypically similar to those that accumulate during wound healing. Owing to their immediate proximity, stromal cells are inevitably affected by the metabolic activity of their cancerous neighbors. Until recently, a role for tumor cell metabolism in influencing the cell fate decisions of neighboring stromal cells has been underappreciated. Here, we propose that metabolites consumed and released by tumor cells act as paracrine factors that regulate the non-malignant cellular composition of a developing tumor by driving stromal cells toward a regenerative response that supports tumor growth.
Keywords: cancer metabolism; cancer-associated fibroblasts; effector T cells; metabolism; regeneration; regulatory T cells; tumor microenvironment; tumor-associated macrophages; wound healing.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest
C.B.T. is a founder of Agios Pharmaceuticals and a member of its scientific advisory board. He is a former member of the Board of Directors and a stockholder of both Merck and Charles River Laboratories. He is a named inventor on patents related to cellular metabolism.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

