The effect of oral contraceptive pill cessation on hepatocellular adenoma diameter: A retrospective cohort study
- PMID: 30773766
- PMCID: PMC6593966
- DOI: 10.1111/liv.14074
The effect of oral contraceptive pill cessation on hepatocellular adenoma diameter: A retrospective cohort study
Abstract
Background & aims: Hepatocellular adenomas (HCA) are rare, hormone-driven, benign liver tumours. HCA >50 mm are associated with haemorrhage and malignant transformation. Guidelines recommend cessation of oral contraceptive pills (OCP) for size reduction; however, it is currently unknown how HCA respond to cessation of OCP. We sought to investigate the effect of OCP cessation on HCA size.
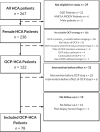
Methods: A retrospective cohort study was performed including HCA patients who stopped OCP intake within 6 months of imaging between 2005 and 2018. Biometrics and hormonal medication use were evaluated with self-designed questionnaires. Response of the largest HCA was evaluated according to Response Evaluation Criteria in Solid Tumours (RECISTv1.1). Cox regression was performed for analysis of factors influencing HCA regression.
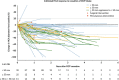
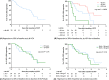
Results: Seventy-eight HCA patients were included, diagnosed at a median (interquartile range) age of 32 (26-41) years. Follow-up was 1.6 (0.4-2.9) years. HCA size at diagnosis ranged 10-167 mm. After a median time of 1.3 (0.6-2.6) years after OCP cessation, 37.2% of HCA showed ≥30% regression, 5.1% complete regression, 56.4% stability and 1.3% progression. No HCA-induced complications were observed during follow-up. Cox regression analysis demonstrated a significant association of HCA size with rate of regression; 50 ≤ HCA < 100 mm (HR 2.4, 95% CI 1.1-5.3; P < 0.05), HCA ≥ 100 mm (HR 8.3, 95% CI 3.3-21.6; P < 0.001).
Conclusions: Ninety-eight per cent of HCA remained stable or regressed after OCP cessation. A longer wait-and-see period was associated with a larger proportion of regressing HCA, without HCA-related complications during follow-up.
Keywords: hepatocellular adenoma; oral contraceptive pill; regression; treatment algorithm.
© 2019 The Authors. Liver International Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not have any disclosures to report.
Figures
Similar articles
-
Regression of hepatocellular adenomas and systemic inflammatory syndrome after cessation of estrogen therapy.Hepatology. 2017 Sep;66(3):989-991. doi: 10.1002/hep.29151. Epub 2017 Jul 18. Hepatology. 2017. PMID: 28295483
-
Retrospective study on timing of resection of hepatocellular adenoma.Br J Surg. 2017 Nov;104(12):1695-1703. doi: 10.1002/bjs.10594. Epub 2017 Aug 30. Br J Surg. 2017. PMID: 28857134
-
Contrast-enhanced ultrasound patterns of hepatocellular adenoma: an Italian multicenter experience.J Ultrasound. 2019 Jun;22(2):157-165. doi: 10.1007/s40477-018-0322-5. Epub 2018 Oct 10. J Ultrasound. 2019. PMID: 30306412 Free PMC article.
-
Management of Hepatocellular Adenoma: Recent Advances.Clin Gastroenterol Hepatol. 2015 Jul;13(7):1221-30. doi: 10.1016/j.cgh.2014.05.023. Epub 2014 Jun 5. Clin Gastroenterol Hepatol. 2015. PMID: 24909909 Review.
-
Regression of multiple hepatocellular adenomas after cessation of oral contraceptive pills: a case report and review of the current literature.Acta Gastroenterol Belg. 2021 Jul-Sep;84(3):505-508. doi: 10.51821/84.3.017. Acta Gastroenterol Belg. 2021. PMID: 34599577 Review.
Cited by
-
Update on the Pathology of Pediatric Liver Tumors: A Pictorial Review.Diagnostics (Basel). 2023 Nov 24;13(23):3524. doi: 10.3390/diagnostics13233524. Diagnostics (Basel). 2023. PMID: 38066766 Free PMC article. Review.
-
From an Incidental Finding to an Emergent Treatment: A Case Report of a Hepatic Adenomatosis and Large Ruptured Hepatic Adenoma.Case Rep Gastrointest Med. 2021 Jun 19;2021:9963440. doi: 10.1155/2021/9963440. eCollection 2021. Case Rep Gastrointest Med. 2021. PMID: 34239741 Free PMC article.
-
A Scoping Review of the Classification, Diagnosis, and Management of Hepatic Adenomas.J Gastrointest Surg. 2022 Apr;26(4):965-978. doi: 10.1007/s11605-022-05246-8. Epub 2022 Jan 26. J Gastrointest Surg. 2022. PMID: 35083725
-
Estrobolome and Hepatocellular Adenomas-Connecting the Dots of the Gut Microbial β-Glucuronidase Pathway as a Metabolic Link.Int J Mol Sci. 2023 Nov 7;24(22):16034. doi: 10.3390/ijms242216034. Int J Mol Sci. 2023. PMID: 38003224 Free PMC article. Review.
-
Safety of hormonal contraception among women with liver disease: an updated systematic review.Contraception. 2025 Jul 7:111012. doi: 10.1016/j.contraception.2025.111012. Online ahead of print. Contraception. 2025. PMID: 40633896 Free PMC article. Review.
References
-
- Rooks JB, Ory HW, Ishak KG, et al. Epidemiology of hepatocellular adenoma. JAMA. 1979;242:644. - PubMed
-
- Edmondson HA, Henderson B, Benton B. Liver‐cell adenomas associated with use of oral contraceptives. N Engl J Med. 1976;294:470‐472. - PubMed
-
- Nault JC, Bioulac‐Sage P, Zucman‐Rossi J. Hepatocellular benign tumors‐from molecular classification to personalized clinical care. Gastroenterology. 2013;144:888‐902. - PubMed
-
- Dokmak S, Paradis V, Vilgrain V, et al. A single‐center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698‐1705. - PubMed
-
- Van Aalten SM, De Man RA, Ijzermans J, Terkivatan T. Systematic review of haemorrhage and rupture of hepatocellular adenomas. Br J Surg. 2012;99:911‐916. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

