Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process
- PMID: 30774334
- PMCID: PMC6361412
- DOI: 10.2147/IJN.S190354
Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process
Erratum in
-
Erratum: Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process [Corrigendum].Int J Nanomedicine. 2019 Aug 23;14:6733-6734. doi: 10.2147/IJN.S208723. eCollection 2019. Int J Nanomedicine. 2019. PMID: 31507317 Free PMC article.
Abstract
Background: Most of nanoparticles are nontoxic and have high absorption capability. Therefore, nanoparticles binding can effectively restrain fibrillation of β-amyloid and α-synuclein proteins and eventually prevent the toxicity of pathogenesis peptide of Alzheimer. Super paramagnetic iron oxide nanoparticles (SPIONs) contain iron oxide core which can be connected to a special part through magnetic coating.
Materials and methods: In this study, the effect of SPIONs with different charges was simultaneously examined on the fibrillation of both β-amyloid and α-synuclein proteins by applying Thioflavin-T assay.
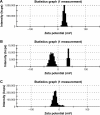
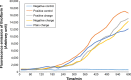
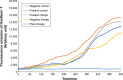
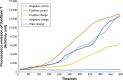
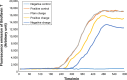
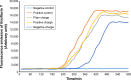
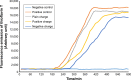
Results: According to the results of the investigation on amyloid-fibrillation mechanism in both β-amyloids and α-synucleins, it was revealed that negatively-charged nanoparticles encoded to -COOH by dextran-coating were able to have a considerable absorption decrease from 17,000-12,000 after 320 minutes delay to lag phase and decrease in binding level of thioflavin-T particles to β-sheets.
Conclusion: The different concentrations of these nanoparticles and special coating of each particle had an effect on the kinetics of β-amyloid and α-synuclein fibrillations.
Keywords: Alzheimer’s disease; Parkinson’s disease; SPION; fibrillation; α-synuclein; β-amyloid.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures

References
-
- Fink AL. Protein aggregation: folding aggregates, inclusion bodies and amyloid. Fold Des. 1998;3(1):R9–R23. - PubMed
-
- Manno M, Mauro M, Craparo EF, Podest A, et al. Kinetics of different processes in human insulin amyloid formation. J Mol Biol. 2007;366(1):258–274. - PubMed
-
- Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rote-none exposure causes highly selective dopaminergic degeneration and α-synuclein aggregation. Experimental Neurology. 2003;179(1):9–16. - PubMed
-
- Cleary JP, Walsh DM, Hofmeister JJ, et al. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8(1):79–84. - PubMed
-
- Walsh DM, Hartley DM, Kusumoto Y, et al. Amyloid beta-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274(36):25945–25952. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

