Profile of plecanatide in the treatment of chronic idiopathic constipation: design, development, and place in therapy
- PMID: 30774407
- PMCID: PMC6348976
- DOI: 10.2147/CEG.S145668
Profile of plecanatide in the treatment of chronic idiopathic constipation: design, development, and place in therapy
Abstract
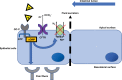
Constipation is a multifactorial disorder that can cause significant psychological distress to patients and economic burden on the health care system. Many patients are not satisfied with their current established treatment, highlighting the need for new and improved therapeutic options. Guanylate cyclase-C (GC-C)/cyclic guanosine monophosphate agonists have emerged as a safe and efficacious class of drugs for the treatment of chronic idiopathic constipation (CIC). Plecanatide, a second-in-class, US FDA-approved, synthetic GC-C agonist, has recently been approved in the US for the treatment of irritable bowel syndrome with constipation at doses of 3 and 6 mg and CIC at the 3 mg dosage. In this study, we summarize the design of this novel 16-amino acid uroguanylin analog, drug development through Phase I, II, and III clinical studies, and its role in the treatment of CIC.
Keywords: constipation; plecanatide; uroguanylin.
Conflict of interest statement
Disclosure AS has served as an advisory board member for Ironwood and Synergy Pharmaceuticals. SSCR has served as an advisory board member and received research grant support from Forest Laboratories, Ironwood Pharmaceuticals, and Synergy Pharmaceuticals. The authors report no other conflicts of interest in this work.
Figures
References
-
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99(4):750–759. - PubMed
-
- Sonnenberg A, Koch TR. Physician visits in the United States for constipation: 1958 to 1986. Dig Dis Sci. 1989;34(4):606–611. - PubMed
-
- Rao SS. Constipation: evaluation and treatment of colonic and anorectal motility disorders. Gastroenterol Clin North Am. 2007;36(3):687–711. - PubMed
-
- Rao SS, Seaton K, Miller MJ, et al. Psychological profiles and quality of life differ between patients with dyssynergia and those with slow transit constipation. J Psychosom Res. 2007;63(4):441–449. - PubMed
-
- Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011;33(8):895–901. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

