Implementation of sorafenib treatment for advanced hepatocellular carcinoma: an illustration of current practice in Taiwan
- PMID: 30774429
- PMCID: PMC6349081
- DOI: 10.2147/CMAR.S186678
Implementation of sorafenib treatment for advanced hepatocellular carcinoma: an illustration of current practice in Taiwan
Abstract
Background: Sorafenib is the first regimen listed in the treatment algorithm for hepatocellular carcinoma (HCC) worldwide. This study aimed to assess the efficacy of sorafenib treatment for advanced HCC in a clinical practice using a nationwide population study.
Methods: All patients registered with a diagnosis of primary HCC and identified as having been prescribed sorafenib between August 2012 and December 2015 were selected from a national database and retrospectively reviewed. Outcomes related to prescription of sorafenib for these patients were further assessed.
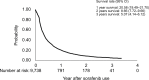
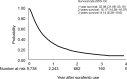
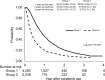
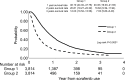
Results: A total of 9,738 patients were enrolled and analyzed. As a result, 32.33% of patients had an initial treatment response and were eligible for the prescribed second term (240 tablets/ term) of sorafenib and 8.91% of patients received more than three terms of sorafenib. Meanwhile, the duration of sorafenib usage beyond 6 months was noted in 15.49% of patients, including 10.59% of patients with a period of usage between 6 and 12 months and 4.9% of patients with more than 12 months usage. Survival analysis showed that patients who received locoregional therapy plus sorafenib had significantly better survival rates than those who underwent only sorafenib treatment. Certain patients who underwent hepatectomy (n=12) or liver transplantation (n=13) were subsequently free of HCC.
Conclusion: The disease control rate of sorafenib in advanced HCC patients in this study seemed similarly poorer as what has been previously reported by clinical trials. The combination of sorafenib and additional treatments could perhaps provide survival benefits and possibly cure disease in combination with surgical management.
Keywords: hepatectomy; hepatocellular carcinoma; liver transplantation; locoregional therapy; outcome; sorafenib.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(2):119–126. - PubMed
-
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. - PubMed
-
- Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma – an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23(11):1535–1547. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. - PubMed
-
- Chang YS, Adnane J, Trail PA, et al. Sorafenib (Bay 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol. 2007;59(5):561–574. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

