Rivaroxaban for venous thromboembolism prevention after major orthopedic surgery: translating trial data into routine clinical practice
- PMID: 30774472
- PMCID: PMC6209349
- DOI: 10.2147/ORR.S105227
Rivaroxaban for venous thromboembolism prevention after major orthopedic surgery: translating trial data into routine clinical practice
Abstract
An established standard of care for the prevention of venous thromboembolism after major orthopedic surgery has been subcutaneous low-molecular-weight heparin. The non-vitamin K antagonist oral anticoagulant rivaroxaban has demonstrated superior efficacy and similar safety to all tested regimens of enoxaparin in large Phase III clinical studies of venous thromboembolism prevention after elective hip and knee arthroplasty. Despite regulatory approval of rivaroxaban for this indication, concerns remain among physicians regarding its optimal and effective use in routine clinical practice. Real-life studies, such as XAMOS and ORTHO-TEP, are providing physicians with more information on the routine use of rivaroxaban for venous thromboembolism prevention after orthopedic surgery, helping to establish its safety and effectiveness in everyday clinical care. Among the most important issues are the risk of bleeding complications, wound healing, timing of first dose, impact of type of anesthesia on thromboprophylaxis effectiveness, patient comorbidities and comedication use, periprocedural management, associated costs, and clinical outcomes in trauma-related fractures. Many of these issues are difficult to study in randomized, double-blind, Phase III trials, and can be assessed more readily using real-life data. In particular, real-life or noninterventional studies lack many of the strict inclusion and exclusion criteria associated with Phase III trials and involve unselected patients who often present with significant comorbidities or comedication use.
Keywords: anticoagulants; arthroplasty; orthopedics; rivaroxaban; thrombosis.
Conflict of interest statement
Disclosure JBW has received honoraria and research support from Bayer HealthCare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, and Pfizer. PM has been a consultant for and has received honoraria from Bayer HealthCare, Sanofi, and Bristol-Myers Squibb. AGGT has been a consultant for Bayer HealthCare, Janssen Pharmaceutical Research and Development LLC, Astellas, Portola, and Takeda. The authors report no other conflicts of interest in this work.
Figures
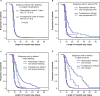
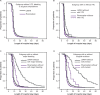
References
-
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians evidence-based clinical practice guidelines (8th edition) Chest. 2008;133(6 Suppl):381S–453S. - PubMed
-
- Hamidi V, Ringerike T, Hagen G, Reikvam A, Klemp M. New anticoagulants as thromboprophylaxis after total hip or knee replacement. Int J Technol Assess Health Care. 2013;29(3):234–243. - PubMed
-
- Friedman RJ. New oral anticoagulants for thromboprophylaxis after total hip or knee arthroplasty. Orthopedics. 2009;32(12 Suppl):79–84. - PubMed
-
- Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358(26):2765–2775. - PubMed
-
- Kakkar AK, Brenner B, Dahl OE, et al. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372(9632):31–39. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

