Zinc Improves Functional Recovery by Regulating the Secretion of Granulocyte Colony Stimulating Factor From Microglia/Macrophages After Spinal Cord Injury
- PMID: 30774583
- PMCID: PMC6367229
- DOI: 10.3389/fnmol.2019.00018
Zinc Improves Functional Recovery by Regulating the Secretion of Granulocyte Colony Stimulating Factor From Microglia/Macrophages After Spinal Cord Injury
Abstract
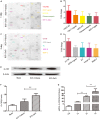
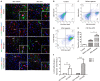
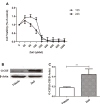
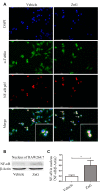
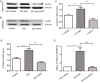
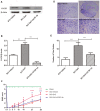
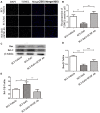
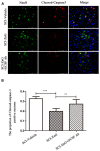
While zinc promotes motor function recovery after spinal cord injury (SCI), the precise mechanisms involved are not fully understood. The present study aimed to elucidate the effects of zinc and granulocyte colony stimulating factor (G-CSF) on neuronal recovery after SCI. The SCI model was established by Allen's method. Injured animals were given glucose and zinc gluconate (ZnG; 30 mg/kg) for the first time at 2 h after injury, the same dose was given for 3 days. A cytokine antibody array was used to screen changes in inflammation at the site of SCI lesion. Immunofluorescence was used to detect the distribution of cytokines. Magnetic beads were also used to isolate cells from the site of SCI lesion. We then investigated the effect of Zinc on apoptosis after SCI by Transferase UTP Nick End Labeling (TUNEL) staining and Western Blotting. Basso Mouse Scale (BMS) scores and immunofluorescence were employed to investigate neuronal apoptosis and functional recovery. We found that the administration of zinc significantly increased the expression of 19 cytokines in the SCI lesion. Of these, G-CSF was shown to be the most elevated cytokine and was secreted by microglia/macrophages (M/Ms) via the nuclear factor-kappa B (NF-κB) signaling pathway after SCI. Increased levels of G-CSF at the SCI lesion reduced the level of neuronal apoptosis after SCI, thus promoting functional recovery. Collectively, our results indicate that the administration of zinc increases the expression of G-CSF secreted by M/Ms, which then leads to reduced levels of neuronal apoptosis after SCI.
Keywords: G-CSF; NF-kappa B; microglia/macrophages; spinal cord injury; zinc.
Figures
References
-
- Bernstein H. G., Ansorge S., Aurin H., Mielke K., Preusser Y., Weiss J., et al. (1986). Immunohistochemical evidence of thiol-protein disulfide oxidoreductase (TPO) in neurosecretory nerve cells of different vertebrates. Cell. Mol. Biol. 32, 37–40. - PubMed
LinkOut - more resources
Full Text Sources

