The Left and Right Ventricles Respond Differently to Variation of Pacing Delays in Cardiac Resynchronization Therapy: A Combined Experimental- Computational Approach
- PMID: 30774598
- PMCID: PMC6367498
- DOI: 10.3389/fphys.2019.00017
The Left and Right Ventricles Respond Differently to Variation of Pacing Delays in Cardiac Resynchronization Therapy: A Combined Experimental- Computational Approach
Abstract
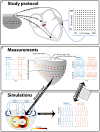
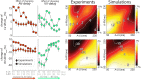
Introduction: Timing of atrial, right (RV), and left ventricular (LV) stimulation in cardiac resynchronization therapy (CRT) is known to affect electrical activation and pump function of the LV. In this study, we used computer simulations, with input from animal experiments, to investigate the effect of varying pacing delays on both LV and RV electrical dyssynchrony and contractile function. Methods: A pacing protocol was performed in dogs with atrioventricular block (N = 6), using 100 different combinations of atrial (A)-LV and A-RV pacing delays. Regional LV and RV electrical activation times were measured using 112 electrodes and LV and RV pressures were measured with catheter-tip micromanometers. Contractile response to a pacing delay was defined as relative change of the maximum rate of LV and RV pressure rise (dP/dtmax) compared to RV pacing with an A-RV delay of 125 ms. The pacing protocol was simulated in the CircAdapt model of cardiovascular system dynamics, using the experimentally acquired electrical mapping data as input. Results: Ventricular electrical activation changed with changes in the amount of LV or RV pre-excitation. The resulting changes in dP/dtmax differed markedly between the LV and RV. Pacing the LV 10-50 ms before the RV led to the largest increases in LV dP/dtmax. In contrast, RV dP/dtmax was highest with RV pre-excitation and decreased up to 33% with LV pre-excitation. These opposite patterns of changes in RV and LV dP/dtmax were reproduced by the simulations. The simulations extended these observations by showing that changes in steady-state biventricular cardiac output differed from changes in both LV and RV dP/dtmax. The model allowed to explain the discrepant changes in dP/dtmax and cardiac output by coupling between atria and ventricles as well as between the ventricles. Conclusion: The LV and the RV respond in a opposite manner to variation in the amount of LV or RV pre-excitation. Computer simulations capture LV and RV behavior during pacing delay variation and may be used in the design of new CRT optimization studies.
Keywords: CircAdapt; cardiac resynchronization therapy; computer simulation; dyssynchrony; hemodynamics; optimization; right ventricle; therapy optimization studies.
Figures





References
-
- Auger D., Hoke U., Bax J. J., Boersma E., Delgado V. (2013). Effect of atrioventricular and ventriculoventricular delay optimization on clinical and echocardiographic outcomes of patients treated with cardiac resynchronization therapy: a meta-analysis. Am. Heart J. 166 20–29. 10.1016/j.ahj.2013.03.021 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

