The Long Pentraxin PTX3 Is of Major Importance Among Acute Phase Proteins in Chickens
- PMID: 30774632
- PMCID: PMC6367253
- DOI: 10.3389/fimmu.2019.00124
The Long Pentraxin PTX3 Is of Major Importance Among Acute Phase Proteins in Chickens
Abstract
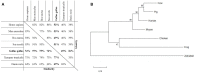
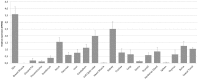
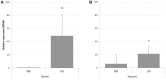
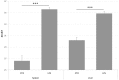
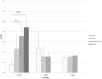
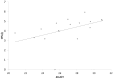
The expression level of acute phase proteins (APPs) mirrors the health status of an individual. In human medicine, C-reactive protein (CRP), and other members of the pentraxin family are of significant relevance for assessing disease severity and prognosis. In chickens, however, which represent the most common livestock species around the world, no such marker has yet gained general acceptance. The aim of this study was therefore, to characterize chicken pentraxin 3 (chPTX3) and to evaluate its applicability as a general marker for inflammatory conditions. The mammalian and chicken PTX3 proteins were predicted to be similar in sequence, domain organization and polymeric structure. Nevertheless, some characteristics like certain sequence sections, which have varied during the evolution of mammals, and species-specific glycosylation patterns, suggest distinct biological functions. ChPTX3 is constitutively expressed in various tissues but, interestingly, could not be found in splenic tissue samples without stimulation. However, upon treatment with lipopolysaccharide (LPS), PTX3 expression in chicken spleens increased to 95-fold within hours. A search for PTX3 reads in various publicly available RNA-seq data sets of chicken spleen and bursa of Fabricius also showed that PTX3 expression increases within days after experimental infection with viral and bacterial pathogens. An experimental infection with avian pathogenic E.coli and qPCR analysis of spleen samples further established a challenge dose-dependent significant up-regulation of chPTX3 in subclinically infected birds of up to over 150-fold as compared to untreated controls. Our results indicate the potential of chPTX3 as an APP marker to monitor inflammatory conditions in poultry flocks.
Keywords: LPS; acute phase proteins; avian pathogenic E. coli; chicken; inflammation; next generation sequencing; pentraxin.
Figures


Similar articles
-
The long pentraxin PTX3 as a prototypic humoral pattern recognition receptor: interplay with cellular innate immunity.Immunol Rev. 2009 Jan;227(1):9-18. doi: 10.1111/j.1600-065X.2008.00719.x. Immunol Rev. 2009. PMID: 19120471 Review.
-
Production of the long pentraxin PTX3 in advanced atherosclerotic plaques.Arterioscler Thromb Vasc Biol. 2002 May 1;22(5):e10-4. doi: 10.1161/01.atv.0000015595.95497.2f. Arterioscler Thromb Vasc Biol. 2002. PMID: 12006411
-
The yin-yang of long pentraxin PTX3 in inflammation and immunity.Immunol Lett. 2014 Sep;161(1):38-43. doi: 10.1016/j.imlet.2014.04.012. Epub 2014 May 1. Immunol Lett. 2014. PMID: 24792672 Free PMC article. Review.
-
Multimer formation and ligand recognition by the long pentraxin PTX3. Similarities and differences with the short pentraxins C-reactive protein and serum amyloid P component.J Biol Chem. 1997 Dec 26;272(52):32817-23. doi: 10.1074/jbc.272.52.32817. J Biol Chem. 1997. PMID: 9407058
-
Analysis of glial secretome: the long pentraxin PTX3 modulates phagocytic activity of microglia.J Neuroimmunol. 2010 Dec 15;229(1-2):63-72. doi: 10.1016/j.jneuroim.2010.07.001. Epub 2010 Jul 31. J Neuroimmunol. 2010. PMID: 20674043
Cited by
-
Cytokine and acute-phase proteins response following vaccination against infectious bronchitis in broilers.Vet Med Sci. 2024 Sep;10(5):e1586. doi: 10.1002/vms3.1586. Vet Med Sci. 2024. PMID: 39171612 Free PMC article.
-
Acute phase proteins patterns as biomarkers in bacterial infection: Recent insights.Open Vet J. 2024 Oct;14(10):2539-2550. doi: 10.5455/OVJ.2024.v14.i10.4. Epub 2024 Oct 31. Open Vet J. 2024. PMID: 39545194 Free PMC article. Review.
-
Transcriptomic Profiles of Pectoralis major Muscles Affected by Spaghetti Meat and Woody Breast in Broiler Chickens.Animals (Basel). 2024 Jan 5;14(2):176. doi: 10.3390/ani14020176. Animals (Basel). 2024. PMID: 38254345 Free PMC article.
-
Health monitoring in birds using bio-loggers and whole blood transcriptomics.Sci Rep. 2021 May 24;11(1):10815. doi: 10.1038/s41598-021-90212-8. Sci Rep. 2021. PMID: 34031452 Free PMC article.
-
Analysis of the Progeny of Sibling Matings Reveals Regulatory Variation Impacting the Transcriptome of Immune Cells in Commercial Chickens.Front Genet. 2019 Nov 14;10:1032. doi: 10.3389/fgene.2019.01032. eCollection 2019. Front Genet. 2019. PMID: 31803225 Free PMC article.
References
-
- Dowton SB, Colten HR. Acute phase reactants in inflammation and infection. Semin Hematol. (1988) 25:84–90. - PubMed
-
- O'Reilly EL, Eckersall PD. Acute phase proteins: a review of their function, behaviour and measurement in chickens. Worlds Poult Sci J. (2014) 70:27–44. 10.1017/S0043933914000038 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

