Deficiency in IL-33/ST2 Axis Reshapes Mitochondrial Metabolism in Lipopolysaccharide-Stimulated Macrophages
- PMID: 30774633
- PMCID: PMC6367255
- DOI: 10.3389/fimmu.2019.00127
Deficiency in IL-33/ST2 Axis Reshapes Mitochondrial Metabolism in Lipopolysaccharide-Stimulated Macrophages
Abstract
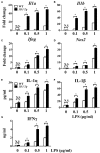
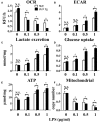
The polarization and function of macrophages play essential roles in controlling immune responses. Interleukin (IL)-33 is a member of the IL-1 family that has been shown to influence macrophage activation and polarization, but the underlying mechanisms are not fully understood. Mitochondrial metabolism has been reported to be a central player in shaping macrophage polarization; previous studies have shown that both aerobic glycolysis and oxidative phosphorylation uniquely regulate the functions of M1 and M2 macrophages. Whether IL-33 polarizes macrophages by reshaping mitochondrial metabolism requires further investigation. In this work, we examined the mitochondrial metabolism of bone marrow-derived macrophages (BMDMs) from either wild type (WT), Il33-overexpressing, or IL-33 receptor knockout (St2-/-) mice challenged with lipopolysaccharide (LPS). We found that after LPS stimulation, compared with WT BMDMs, St2-/- BMDMs had reduced cytokine production and increased numbers and activity of mitochondria via the metabolism regulator peroxisome proliferator-activated receptor-C coactivator-1 α (PGC1α). This was demonstrated by increased mitochondrial DNA copy number, mitochondria counts, mitochondria fission- and fusion-related gene expression, oxygen consumption rates, and ATP production, and decreased glucose uptake, lactate production, and extracellular acidification rates. For Il33-overexpressing BMDMs, the metabolic reprogramming upon LPS stimulation was similar to WT BMDMs, and was accompanied by increased M1 macrophage activity. Our findings suggested that the pleiotropic IL-33/ST2 pathway may influence the polarization and function of macrophages by regulating mitochondrial metabolism.
Keywords: ATP; IL-33; PGC1α; ST2; macrophage.
Figures





References
-
- Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, et al. Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. (2015) 21:65–80. 10.1016/j.cmet.2014.12.005 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

