Immune Cell Composition in Human Non-small Cell Lung Cancer
- PMID: 30774636
- PMCID: PMC6367276
- DOI: 10.3389/fimmu.2018.03101
Immune Cell Composition in Human Non-small Cell Lung Cancer
Abstract
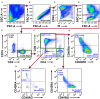
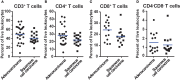
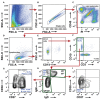
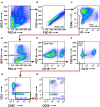
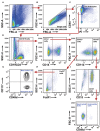
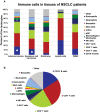
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death in the world. Immunological analysis of the tumor microenvironment (immunoscore) shows great promise for improved prognosis and prediction of response to immunotherapy. However, the exact immune cell composition in NSCLC remains unclear. Here, we used flow cytometry to characterize the immune infiltrate in NSCLC tumors, non-cancerous lung tissue, regional lymph node, and blood. The cellular identity of >95% of all CD45+ immune cells was determined. Thirteen distinct immune cell types were identified in NSCLC tumors. T cells dominated the lung cancer landscape (on average 47% of all CD45+ immune cells). CD4+ T cells were the most abundant T cell population (26%), closely followed by CD8+ T cells (22%). Double negative CD4-CD8- T cells represented a small fraction (1.4%). CD19+ B cells were the second most common immune cell type in NSCLC tumors (16%), and four different B cell sub-populations were identified. Macrophages and natural killer (NK) cells composed 4.7 and 4.5% of the immune cell infiltrate, respectively. Three types of dendritic cells (DCs) were identified (plasmacytoid DCs, CD1c+ DCs, and CD141+ DCs) which together represented 2.1% of all immune cells. Among granulocytes, neutrophils were frequent (8.6%) with a high patient-to-patient variability, while mast cells (1.4%), basophils (0.4%), and eosinophils (0.3%) were less common. Across the cohort of patients, only B cells showed a significantly higher representation in NSCLC tumors compared to the distal lung. In contrast, the percentages of macrophages and NK cells were lower in tumors than in non-cancerous lung tissue. Furthermore, the fraction of macrophages with high HLA-DR expression levels was higher in NSCLC tumors relative to distal lung tissue. To make the method readily accessible, antibody panels and flow cytometry gating strategy used to identify the various immune cells are described in detail. This work should represent a useful resource for the immunomonitoring of patients with NSCLC.
Keywords: NSCLC; flow cytometry; human lung cancer; immunomonitoring; immunoscore; tumor-infiltrating immune cells.
Figures







References
-
- Travis WD, Brambilla E, Noguchi M, Nicholson AG, Geisinger K, Yatabe Y, et al. . International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proc Am Thorac Soc. (2011) 8:381. 10.1513/pats.201107-042ST - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

