The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression
- PMID: 30774637
- PMCID: PMC6367985
- DOI: 10.3389/fimmu.2018.03181
The Human Endolymphatic Sac and Inner Ear Immunity: Macrophage Interaction and Molecular Expression
Abstract
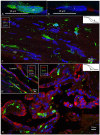
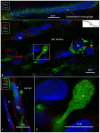
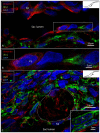
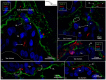
Background: The endolymphatic sac (ES) is endowed with a multitude of white blood cells that may trap and process antigens that reach the inner ear from nearby infection-prone areas, it thus serves as an immunologic defense organ. The human ES, and unexpectedly the rest of the inner ear, has been recently shown to contain numerous resident macrophages. In this paper, we describe ES macrophages using super-resolution structured fluorescence microscopy (SR-SIM) and speculate on these macrophages' roles in human inner ear defense. Material and Methods: After ethical permission was obtained, human vestibular aqueducts were collected during trans-labyrinthine surgery for acoustic neuroma removal. Tissues were placed in fixative before being decalcified, rapidly frozen, and cryostat sectioned. Antibodies against IBA1, cytokine fractalkine (CX3CL1), toll-like receptor 4 (TLR4), cluster of differentiation (CD)68, CD11b, CD4, CD8, and the major histocompatibility complex type II (MHCII) were used for immunohistochemistry. Results: A large number of IBA1-positive cells with different morphologies were found to reside in the ES; the cells populated surrounding connective tissue and the epithelium. Macrophages interacted with other cells, showed migrant behavior, and expressed immune cell markers, all of which suggest their active role in the innate and adaptive inner ear defense and tolerance. Discussion: High-resolution immunohistochemistry shows that antigens reaching the ear may be trapped and processed by an immune cell machinery located in the ES. Thereby inflammatory activity may be evaded near the vulnerable inner ear sensory structures. We speculate on the immune defensive link between the ES and the rest of the inner ear.
Keywords: IBA1; cochlea; human; macrophages; structured illumination microscopy.
Figures

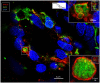
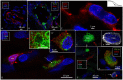

Similar articles
-
Human Inner Ear Immune Activity: A Super-Resolution Immunohistochemistry Study.Front Neurol. 2019 Jul 10;10:728. doi: 10.3389/fneur.2019.00728. eCollection 2019. Front Neurol. 2019. PMID: 31354608 Free PMC article.
-
Immunodefence of the inner ear? Lymphocyte-macrophage interaction in the endolymphatic sac.Acta Otolaryngol. 1980 Mar-Apr;89(3-4):283-94. doi: 10.3109/00016488009127140. Acta Otolaryngol. 1980. PMID: 7395499
-
Macrophages in the Human Cochlea: Saviors or Predators-A Study Using Super-Resolution Immunohistochemistry.Front Immunol. 2018 Feb 13;9:223. doi: 10.3389/fimmu.2018.00223. eCollection 2018. Front Immunol. 2018. PMID: 29487598 Free PMC article.
-
Inner ear immunity.Hear Res. 2022 Jun;419:108518. doi: 10.1016/j.heares.2022.108518. Epub 2022 May 11. Hear Res. 2022. PMID: 35584985 Review.
-
Distribution of Immune Cells Including Macrophages in the Human Cochlea.Front Neurol. 2021 Nov 22;12:781702. doi: 10.3389/fneur.2021.781702. eCollection 2021. Front Neurol. 2021. PMID: 34880828 Free PMC article. Review.
Cited by
-
A Micro-CT and Synchrotron Imaging Study of the Human Endolymphatic Duct with Special Reference to Endolymph Outflow and Meniere's Disease.Sci Rep. 2020 May 19;10(1):8295. doi: 10.1038/s41598-020-65110-0. Sci Rep. 2020. PMID: 32427861 Free PMC article.
-
Density of Macrophages Immunostained With Anti-iba1 Antibody in the Vestibular Endorgans After Cochlear Implantation in the Human.Otol Neurotol. 2019 Sep;40(8):e774-e781. doi: 10.1097/MAO.0000000000002313. Otol Neurotol. 2019. PMID: 31335797 Free PMC article.
-
Differential Proinflammatory Signature in Vestibular Migraine and Meniere Disease.Front Immunol. 2019 Jun 4;10:1229. doi: 10.3389/fimmu.2019.01229. eCollection 2019. Front Immunol. 2019. PMID: 31214186 Free PMC article.
-
Inhalation of Molecular Hydrogen, a Rescue Treatment for Noise-Induced Hearing Loss.Front Cell Neurosci. 2021 Jun 1;15:658662. doi: 10.3389/fncel.2021.658662. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34140880 Free PMC article.
-
Age-Related Changes in Immune Cells of the Human Cochlea.Front Neurol. 2019 Aug 16;10:895. doi: 10.3389/fneur.2019.00895. eCollection 2019. Front Neurol. 2019. PMID: 31474935 Free PMC article.
References
-
- O'Malley JT, Nadol JB, Jr, McKenna MJ. Anti CD163+, Iba1+, and CD68+ Cells in the adult human inner ear: normal distribution of an unappreciated class of macrophages/microglia and implications for inflammatory otopathology in humans. Otol Neurotol. (2016) 37:99–108. 10.1097/MAO.0000000000000879 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

