Crystallographic characterization of Lu2C2 n (2 n = 76-90): cluster selection by cage size
- PMID: 30774877
- PMCID: PMC6345353
- DOI: 10.1039/c8sc03886d
Crystallographic characterization of Lu2C2 n (2 n = 76-90): cluster selection by cage size
Abstract
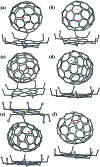
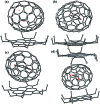
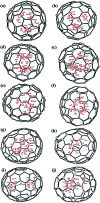
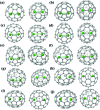
The successful isolation and unambiguous crystallographic assignment of a series of lutetium-containing endohedral metallofullerenes (EMFs), Lu2C2n (2n = 76, 78, 80, 84, 86, 88, 90), reveal an unrecognized decisive effect of the cage size on the configuration of the encapsulated clusters. The molecular structures of these compounds are unambiguously assigned as Lu2@T d(2)-C76, Lu2@D 3h(5)-C78, Lu2@C 2v(5)-C80, Lu2@C 2v(7)-C84, Lu2@C s(8)-C86, Lu2@C s(15)-C86, Lu2@C 1(26)-C88, Lu2C2@C 2v(9)-C86, Lu2C2@C s(32)-C88 and Lu2C2@D 2(35)-C88. Specifically, when the cage is relatively small, Lu2@C2n (2n = 76-86) are all dimetallofullerenes (di-EMFs) and a Lu-Lu single bond could be formed between the two lutetium ions inside the cages. However, when the cage expands further, the valence electrons forming the possible Lu-Lu bond donate to a readily inserted C2-unit, resulting in the formation of carbide EMFs, Lu2C2@C2n (2n = 86, 88). Consistently, our theoretical results reveal that all these EMFs are thermodynamically favorable isomers. Thus the comprehensive characterization of the series of Lu2C76-90 isomers and the overall agreement between the experimental and theoretical results reveal for the first time that the exact configuration of the internal metallic cluster is determined by the cage size, taking a solid step towards the controlled synthesis of novel hybrid molecules which may have potential applications as building blocks of single molecule devices.
Figures
Similar articles
-
Lu2@C2n (2n = 82, 84, 86): Crystallographic Evidence of Direct Lu-Lu Bonding between Two Divalent Lutetium Ions Inside Fullerene Cages.J Am Chem Soc. 2017 Jul 26;139(29):9979-9984. doi: 10.1021/jacs.7b04421. Epub 2017 Jul 17. J Am Chem Soc. 2017. PMID: 28679207
-
Significant Roles of a Particularly Stable Two-Center Two-Electron Lu-Lu σ Bond in Lu2@C86: Electronic Structure of Lu and Radius of Lu2.Inorg Chem. 2021 Feb 15;60(4):2425-2436. doi: 10.1021/acs.inorgchem.0c03336. Epub 2021 Jan 26. Inorg Chem. 2021. PMID: 33497217
-
Isolation and Crystallographic Characterization of Lu3 N@C2n (2n=80-88): Cage Selection by Cluster Size.Chemistry. 2018 Nov 7;24(62):16692-16698. doi: 10.1002/chem.201804651. Epub 2018 Oct 9. Chemistry. 2018. PMID: 30221415
-
Endohedral Metallofullerenes: New Structures and Unseen Phenomena.Chemistry. 2020 May 7;26(26):5748-5757. doi: 10.1002/chem.201905306. Epub 2020 Mar 13. Chemistry. 2020. PMID: 31886563 Review.
-
Structural and electronic properties of endohedral metallofullerenes.Chem Rec. 2012 Apr;12(2):256-69. doi: 10.1002/tcr.201100038. Epub 2012 Apr 10. Chem Rec. 2012. PMID: 22489092 Review.
Cited by
-
Recent advances in endohedral metallofullerenes.Fundam Res. 2023 Dec 29;5(2):767-781. doi: 10.1016/j.fmre.2023.12.004. eCollection 2025 Mar. Fundam Res. 2023. PMID: 40242547 Free PMC article. Review.
-
Stabilizing a three-center single-electron metal-metal bond in a fullerene cage.Chem Sci. 2021 Apr 2;12(20):6890-6895. doi: 10.1039/d1sc00965f. Chem Sci. 2021. PMID: 34123317 Free PMC article.
-
Nd─Nd Bond in Ih and D5h Cage Isomers of Nd2 @C80 Stabilized by Electrophilic CF3 Addition.Adv Sci (Weinh). 2024 Jan;11(1):e2305190. doi: 10.1002/advs.202305190. Epub 2023 Nov 9. Adv Sci (Weinh). 2024. PMID: 37946664 Free PMC article.
-
Trapping an unprecedented Ti3C3 unit inside the icosahedral C80 fullerene: a crystallographic survey.Chem Sci. 2019 Oct 14;10(47):10925-10930. doi: 10.1039/c9sc04315b. eCollection 2019 Dec 21. Chem Sci. 2019. PMID: 32190248 Free PMC article.
-
Single Molecule Magnetism with Strong Magnetic Anisotropy and Enhanced Dy∙∙∙Dy Coupling in Three Isomers of Dy-Oxide Clusterfullerene Dy2O@C82.Adv Sci (Weinh). 2019 Aug 15;6(20):1901352. doi: 10.1002/advs.201901352. eCollection 2019 Oct 16. Adv Sci (Weinh). 2019. PMID: 31637168 Free PMC article.
References
-
- Bao L., Peng P., Lu X. Acc. Chem. Res. 2018;51:810–815. - PubMed
-
- Popov A. A., Yang S., Dunsch L. Chem. Rev. 2013;113:5989–6113. - PubMed
-
- Lu X., Feng L., Akasaka T., Nagase S. Chem. Soc. Rev. 2012;41:7723–7760. - PubMed
-
- Lu X., Akasaka T., Nagase S. Acc. Chem. Res. 2013;46:1627–1635. - PubMed
-
- Cai T., Xu L., Anderson M. R., Ge Z., Zuo T., Wang X., Olmstead M. M., Balch A. L., Gibson H. W., Dorn H. C. J. Am. Chem. Soc. 2006;128:8581–8589. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

