The inverted ketene synthon: a double umpolung approach to enantioselective β2,3-amino amide synthesis
- PMID: 30774911
- PMCID: PMC6349014
- DOI: 10.1039/c8sc04330b
The inverted ketene synthon: a double umpolung approach to enantioselective β2,3-amino amide synthesis
Abstract
A stereocontrolled synthesis of β2,3-amino amides is reported. Innovation is encapsulated by the first use of nitroalkenes to achieve double umpolung in enantioselective β-amino amide synthesis. Step economy is also fulfilled by the use of Umpolung Amide Synthesis (UmAS) in the second step, delivering the amide product without intermediacy of a carboxylic acid or activated derivative. Molybdenum oxide-mediated hydride reduction provides the anti-β2,3-amino amide with high selectivity.
Figures
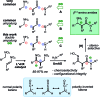
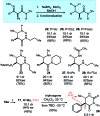
References
-
-
Reviews:
- Seebach D., Matthews J. L. Chem. Commun. 1997:2015–2022.
- Cheng R. P., Gellman S. H., DeGrado W. F. Chem. Rev. 2001;101:3219–3232. - PubMed
- Kiss L., Cherepanova M., Fülöp F. Tetrahedron. 2015;71:2049–2069.
- Ashfaq M., Tabassum R., Ahmad M. M., Hassan N. A., Oku H., Rivera G. Med. Chem. 2015;5:295–309.
-
-
- Aguilar M.-I., Purcell A. W., Devi R., Lew R., Rossjohn J., Smith A. I., Perlmutter P. Org. Biomol. Chem. 2007;5:2884–2890. - PubMed
- Seebach D., Matthews J. L. Chem. Commun. 1997:2015–2022.
-
- Fernando R., Francisco C., Alberto A., Jesus Hector B., Jesus Manuel P., Maria del Mar Z. Curr. Top. Med. Chem. 2014;14:1225–1234. - PubMed
-
- Hook D. F., Bindschädler P., Mahajan Y. R., Šebesta R., Kast P., Seebach D. Chem. Biodiversity. 2005;2:591–632. - PubMed
-
- Kim D., Wang L., Beconi M., Eiermann G. J., Fisher M. H., He H., Hickey G. J., Kowalchick J. E., Leiting B., Lyons K., Marsilio F., McCann M. E., Patel R. A., Petrov A., Scapin G., Patel S. B., Roy R. S., Wu J. K., Wyvratt M. J., Zhang B. B., Zhu L., Thornberry N. A., Weber A. E. J. Med. Chem. 2005;48:141–151. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

