Biophysical basis of skin cancer margin assessment using Raman spectroscopy
- PMID: 30775086
- PMCID: PMC6363200
- DOI: 10.1364/BOE.10.000104
Biophysical basis of skin cancer margin assessment using Raman spectroscopy
Abstract
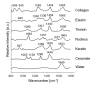
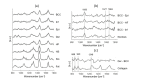
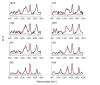
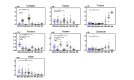
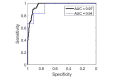
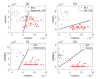
Achieving adequate margins during tumor margin resection is critical to minimize the recurrence rate and maximize positive patient outcomes during skin cancer surgery. Although Mohs micrographic surgery is by far the most effective method to treat nonmelanoma skin cancer, it can be limited by its inherent required infrastructure, including time-consuming and expensive on-site histopathology. Previous studies have demonstrated that Raman spectroscopy can accurately detect basal cell carcinoma (BCC) from surrounding normal tissue; however, the biophysical basis of the detection remained unclear. Therefore, we aim to explore the relevant Raman biomarkers to guide BCC margin resection. Raman imaging was performed on skin tissue samples from 30 patients undergoing Mohs surgery. High correlations were found between the histopathology and Raman images for BCC and primary normal structures (including epidermis, dermis, inflamed dermis, hair follicle, hair shaft, sebaceous gland and fat). A previously developed model was used to extract the biochemical changes associated with malignancy. Our results showed that BCC had a significantly different concentration of nucleus, keratin, collagen, triolein and ceramide compared to normal structures. The nucleus accounted for most of the discriminant power (90% sensitivity, 92% specificity - balanced approach). Our findings suggest that Raman spectroscopy is a promising surgical guidance tool for identifying tumors in the resection margins.
Conflict of interest statement
The authors declare that there are no conflicts of interest related to this article.
Figures

References
-
- Society A. C., Cancer facts & figures 2018 (American Cancer Society, Altanta, 2018).
-
- Mosterd K., Krekels G. A., Nieman F. H., Ostertag J. U., Essers B. A., Dirksen C. D., Steijlen P. M., Vermeulen A., Neumann H., Kelleners-Smeets N. W., “Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up,” Lancet Oncol. 9(12), 1149–1156 (2008). 10.1016/S1470-2045(08)70260-2 - DOI - PubMed
-
- Smeets N. W., Kuijpers D. I., Nelemans P., Ostertag J. U., Verhaegh M. E., Krekels G. A., Neumann H. A., “Mohs’ micrographic surgery for treatment of basal cell carcinoma of the face--results of a retrospective study and review of the literature,” Br. J. Dermatol. 151(1), 141–147 (2004). 10.1111/j.1365-2133.2004.06047.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
