Biomarkers in patients with mucopolysaccharidosis type II and IV
- PMID: 30775257
- PMCID: PMC6365937
- DOI: 10.1016/j.ymgmr.2019.100455
Biomarkers in patients with mucopolysaccharidosis type II and IV
Abstract
Glycosaminoglycans (GAGs), dermatan sulfate (DS), heparan sulfate (HS), and keratan sulfate (KS), are the primary biomarkers in patients with mucopolysaccharidoses (MPS); however, little is known about other biomarkers. To explore potential biomarkers and their correlation with GAGs, blood samples were collected from 46 MPS II patients, 34 MPS IVA patients, and 5 MPS IVB patients. We evaluated the levels of 8 pro-inflammatory factors (EGF, IL-1β, IL-6, MIP-1α, TNF-α, MMP-1, MMP-2, and MMP-9), collagen type II, and DS, HS (HS0S, HSNS), and KS (mono-sulfated, di-sulfated) in blood. Eight biomarkers measured were significantly elevated in untreated MPS II patients, compared with those in normal controls: EGF, IL-1β, IL-6, HS0S, HSNS, DS, mono-sulfated KS, and di-sulfated KS. The same eight biomarkers remained elevated in ERT-treated patients. However, only three biomarkers remained elevated in post-HSCT MPS II patients: EGF, mono-sulfated KS, and di-sulfated KS. Post-HSCT patients with MPS II showed that IL-1β and IL-6 were normalized as HS and DS levels decreased. Eight biomarkers were significantly elevated in untreated MPS IVA patients: EGF, IL-1β, IL-6, MIP-1α, MMP-9, HSNS, mono-sulfated KS, and di-sulfated KS, and four biomarkers were elevated in MPS IVA patients under ERT: IL-6, TNF-α, mono-sulfated KS, and di-sulfated KS. There was no reduction of KS in the ERT-treated MPS IVA patient, compared with untreated patients. Two biomarkers were significantly elevated in untreated MPS IVB patients: IL-6 and TNF-α. Reversely, collagen type II level was significantly decreased in untreated and ERT-treated MPS II patients and untreated MPS IVA patients. In conclusion, selected pro-inflammatory factors can be potential biomarkers in patients with MPS II and IV as well as GAGs levels.
Keywords: Cytokines; Glycosaminoglycans; Hunter syndrome; Inflammation; Morquio syndrome.
Figures
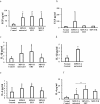


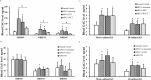
References
-
- Tomatsu S., Kubaski F. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 10, Mucopolysaccharidosis Type II: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 165–209.
-
- Sawamoto K., Alméciga-Díaz C.J. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 12, Mucopolysaccharidosis Type IVA: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 235–271.
-
- Higaki K., Ninomiya H. Vol. 1. Nova Science Publishers; New York: 2018. Chapter 13, Mucopolysaccharidosis Type IVB: Clinical Features, Biochemistry, Diagnosis, Genetics and Treatment, Mucopolysaccharidoses Update vol.1; pp. 273–283.
-
- Simonaro C.M., D'Angelo M., Haskins M.E., Schuchman E.H. Joint and bone disease in mucopolysaccharidoses VI and VII: identification of new therapeutic targets and biomarkers using animal models. Pediatr. Res. 2005;57:701–707. - PubMed
-
- Simonaro C.M. vol. 1. Nova Science Publishers; New York: 2018. Chapter 6, Inflammation and Its Role in the Lysosomal Storage Disorders, Mucopolysaccharidoses Update vol.1; pp. 75–86.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

