The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice
- PMID: 30775438
- PMCID: PMC6365110
- DOI: 10.1126/sciadv.aau8317
The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice
Erratum in
-
Correction for the Research Article: "The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice" by P. Zheng, B. Zeng, M. Liu, J. Chen, J. Pan, Y. Han, Y. Liu, K. Cheng, C. Zhou, H. Wang, X. Zhou, S. Gui, S. W. Perry, M. Wong, J. Licinio, H. Wei, and P. Xie.Sci Adv. 2019 Jun 21;5(6):eaay2759. doi: 10.1126/sciadv.aay2759. eCollection 2019 Jun. Sci Adv. 2019. PMID: 31245543 Free PMC article.
Abstract
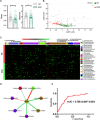
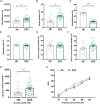
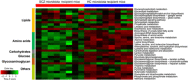
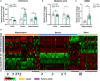
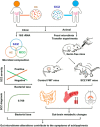
Schizophrenia (SCZ) is a devastating mental disorder with poorly defined underlying molecular mechanisms. The gut microbiome can modulate brain function and behaviors through the microbiota-gut-brain axis. Here, we found that unmedicated and medicated patients with SCZ had a decreased microbiome α-diversity index and marked disturbances of gut microbial composition versus healthy controls (HCs). Several unique bacterial taxa (e.g., Veillonellaceae and Lachnospiraceae) were associated with SCZ severity. A specific microbial panel (Aerococcaceae, Bifidobacteriaceae, Brucellaceae, Pasteurellaceae, and Rikenellaceae) enabled discriminating patients with SCZ from HCs with 0.769 area under the curve. Compared to HCs, germ-free mice receiving SCZ microbiome fecal transplants had lower glutamate and higher glutamine and GABA in the hippocampus and displayed SCZ-relevant behaviors similar to other mouse models of SCZ involving glutamatergic hypofunction. Together, our findings suggest that the SCZ microbiome itself can alter neurochemistry and neurologic function in ways that may be relevant to SCZ pathology.
Figures
References
-
- Long J., Huang G., Liang W., Liang B., Chen Q., Xie J., Jiang J., Su L., The prevalence of schizophrenia in mainland China: Evidence from epidemiological surveys. Acta Psychiatr. Scand. 130, 244–256 (2014). - PubMed
-
- Cryan J. F., Dinan T. G., Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 (2012). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

