A promising approach to targeting type 1 IFN in systemic lupus erythematosus
- PMID: 30776023
- PMCID: PMC6391084
- DOI: 10.1172/JCI127101
A promising approach to targeting type 1 IFN in systemic lupus erythematosus
Abstract
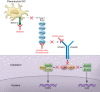
Despite advances in understanding systemic lupus erythematosus (SLE) pathogenesis, most clinical trials of new targeted therapies have been met with disappointment. The type I IFN pathway is believed to play an important role in SLE, and the proposed involvement of this pathway helps explain the frustration behind the failure at targeting either IFN-α or the type 1 IFN receptor itself. In this issue of the JCI, Furie et al. report on an intriguing phase 1b study that demonstrates an approach for inhibiting this pathway in the skin using an mAB (BIIB059) that targets the blood DC antigen 2 (BDCA-2) receptor on plasmacytoid DCs (pDCs). BIIB059 decreased IFN expression and improved cutaneous lupus disease activity, with a favorable safety profile. Whether or not this strategy will be effective in managing SLE in other organs remains unanswered. However, these results suggest that closing the door on targeting the type 1 IFN pathway in SLE may be premature and highlight the emerging question of whether an organ-specific approach toward lupus trials and treatment should be the wave of the future.
Conflict of interest statement
Figures
Comment on
-
Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus.J Clin Invest. 2019 Mar 1;129(3):1359-1371. doi: 10.1172/JCI124466. Epub 2019 Feb 18. J Clin Invest. 2019. PMID: 30645203 Free PMC article. Clinical Trial.
References
-
- van Vollenhoven RF, et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet. 2018;392(10155):1330–1339. doi: 10.1016/S0140-6736(18)32167-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

