Epithelial barrier repair and prevention of allergy
- PMID: 30776025
- PMCID: PMC6436854
- DOI: 10.1172/JCI124608
Epithelial barrier repair and prevention of allergy
Abstract
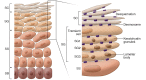
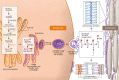
Allergic diseases have in common a dysfunctional epithelial barrier, which allows the penetration of allergens and microbes, leading to the release of type 2 cytokines that drive allergic inflammation. The accessibility of skin, compared with lung or gastrointestinal tissue, has facilitated detailed investigations into mechanisms underlying epithelial barrier dysfunction in atopic dermatitis (AD). This Review describes the formation of the skin barrier and analyzes the link between altered skin barrier formation and the pathogenesis of AD. The keratinocyte differentiation process is under tight regulation. During epidermal differentiation, keratinocytes sequentially switch gene expression programs, resulting in terminal differentiation and the formation of a mature stratum corneum, which is essential for the skin to prevent allergen or microbial invasion. Abnormalities in keratinocyte differentiation in AD skin result in hyperproliferation of the basal layer of epidermis, inhibition of markers of terminal differentiation, and barrier lipid abnormalities, compromising skin barrier and antimicrobial function. There is also compelling evidence for epithelial dysregulation in asthma, food allergy, eosinophilic esophagitis, and allergic rhinosinusitis. This Review examines current epithelial barrier repair strategies as an approach for allergy prevention or intervention.
Conflict of interest statement
Figures

References
-
- Renz H, et al. Food allergy. Nat Rev Dis Primers. 2018;4:17098. - PubMed

