Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis
- PMID: 30776055
- PMCID: PMC6515582
- DOI: 10.1001/jamaneurol.2018.4905
Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis
Abstract
Importance: Uncertainty remains about how aggressively to treat early multiple sclerosis. High-efficacy disease-modifying therapies (DMTs) are often reserved for individuals expressing poor prognostic features at baseline.
Objective: To analyze long-term outcomes in a population-based cohort according to initial treatment strategy.
Design, setting and participants: In this cohort study, data were derived from January 1998 to December 2016, and analysis was performed in January 2017. From a total of 720 patients prescribed a DMT, 592 (82%) were included in analysis. Reasons for exclusion were first treated elsewhere or privately (n = 39), clinical trial participant (n = 25), and insufficient clinical data (n = 45).
Exposures: Patients were classified according to first-line treatment strategy: high-efficacy (early intensive treatment [EIT]) or moderate-efficacy DMT (escalation [ESC]).
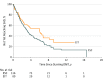
Main outcomes and measures: Primary outcome was 5-year change in Expanded Disability Status Scale score. Secondary outcome was time to sustained accumulation of disability (SAD). Models were adjusted for sex, age at treatment, year of starting DMT, and escalation to high-efficacy treatment in the ESC group.
Results: Mean (SD) age of 592 patients at symptom onset was 27.0 (9.4) years. Mean (SD) 5-year change in Expanded Disability Status Scale score was lower in the EIT group than the ESC group (0.3 [1.5] vs 1.2 [1.5]); this remained significant after adjustment for relevant covariates (β = -0.85; 95% CI, -1.38 to -0.32; P = .002). Median (95% CI) time to SAD was 6.0 (3.17-9.16) years for EIT and 3.14 (2.77-4.00) years for ESC (P = .05). For those within the ESC group who escalated to high-efficacy DMT as second-line treatment, median (95% CI) time to SAD was 3.3 years (1.8-5.6; compared with EIT group log-rank test P = .08). After adjustment for relevant covariates, there was no difference in hazard of SAD between the groups. However, 60% of those who escalated to high-efficacy DMTs were observed to develop SAD while still receiving initial moderate-efficacy treatment before escalation.
Conclusions and relevance: In a real-life setting, long-term outcomes were more favorable following early intensive therapy vs first-line moderate-efficacy DMT. Contemporary surveillance strategies and escalation protocols may be insufficiently responsive. This finding is particularly relevant as patients in real-world practice are typically selected for an EIT approach to therapy on the basis of clinical and radiological features predictive of a poor outcome. These data support the need for a prospective randomized clinical trial.
Conflict of interest statement
Figures

References
-
- Palace J, Duddy M, Bregenzer T, et al. . Effectiveness and cost-effectiveness of interferon beta and glatiramer acetate in the UK Multiple Sclerosis Risk Sharing Scheme at 6 years: a clinical cohort study with natural history comparator. Lancet Neurol. 2015;14(5):497-505. doi:10.1016/S1474-4422(15)00018-6 - DOI - PubMed
-
- Tilling K, Lawton M, Robertson N, et al. . Modelling disease progression in relapsing-remitting onset multiple sclerosis using multilevel models applied to longitudinal data from two natural history cohorts and one treated cohort. Health Technol Assess. 2016;20(81):1-48. doi:10.3310/hta20810. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

