Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants
- PMID: 30776084
- PMCID: PMC6378929
- DOI: 10.1002/14651858.CD004868.pub5
Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants
Update in
-
Late erythropoiesis-stimulating agents to prevent red blood cell transfusion in preterm or low birth weight infants.Cochrane Database Syst Rev. 2020 Jan 28;1(1):CD004868. doi: 10.1002/14651858.CD004868.pub6. Cochrane Database Syst Rev. 2020. PMID: 31990982 Free PMC article.
Abstract
Background: Preterm infants have low plasma levels of erythropoietin (EPO), providing a rationale for the use of erythropoiesis-stimulating agents (ESAs) to prevent or treat anaemia. Darbepoetin (Darbe) and EPO are currently available ESAs.
Objectives: To assess the effectiveness and safety of late initiation of ESAs, between eight and 28 days after birth, in reducing the use of red blood cell (RBC) transfusions in preterm or low birth weight infants.
Search methods: We used the standard search strategy of Cochrane Neonatal to search the Cochrane Central Register of Controlled Trials (CENTRAL 2018, Issue 5), MEDLINE via PubMed (1966 to 5 June 2018), Embase (1980 to 5 June 2018), and CINAHL (1982 to 5 June 2018). We searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi-randomised trials.
Selection criteria: Randomised or quasi-randomised controlled trials of late initiation of EPO treatment (started at ≥ eight days of age) versus placebo or no intervention in preterm (< 37 weeks) or low birth weight (< 2500 grams) neonates.
Data collection and analysis: We performed data collection and analyses in accordance with the methods of the Cochrane Neonatal Review Group. We used the GRADE approach to assess the quality of the evidence.
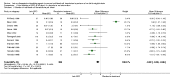
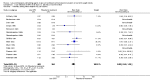
Main results: We include 31 studies (32 comparisons) randomising 1651 preterm infants. Literature searches in 2018 identified one new study for inclusion. No new on-going trials were identified and no studies used darbepoetin.Most included trials were of small sample size. The meta-analysis showed a significant effect on the use of one or more RBC transfusions (21 studies (n = 1202); typical risk ratio (RR) 0.72, 95% confidence interval (CI) 0.65 to 0.79; typical risk difference (RD) -0.17, 95% CI -0.22 to -0.12; typical number needed to treat for an additional beneficial outcome (NNTB) 6, 95% CI 5 to 8). There was moderate heterogeneity for this outcome (RR I² = 66%; RD I² = 58%). The quality of the evidence was very low. We obtained similar results in secondary analyses based on different combinations of high/low doses of EPO and iron supplementation. There was no significant reduction in the total volume (mL/kg) of blood transfused per infant (typical mean difference (MD) -1.6 mL/kg, 95% CI -5.8 to 2.6); 5 studies, 197 infants). There was high heterogeneity for this outcome (I² = 92%). There was a significant reduction in the number of transfusions per infant (11 studies enrolling 817 infants; typical MD -0.22, 95% CI -0.38 to -0.06). There was high heterogeneity for this outcome (I² = 94%).Three studies including 404 infants reported on retinopathy of prematurity (ROP) (all stages or stage not reported), with a typical RR 1.27 (95% CI 0.99 to 1.64) and a typical RD of 0.09 (95% CI -0.00 to 0.18). There was high heterogeneity for this outcome for both RR (I² = 83%) and RD (I² = 82%). The quality of the evidence was very low.Three trials enrolling 442 infants reported on ROP (stage ≥ 3). The typical RR was 1.73 (95% CI 0.92 to 3.24) and the typical RD was 0.05 (95% CI -0.01 to 0.10). There was no heterogeneity for this outcome for RR (I² = 18%) but high heterogeneity for RD (I² = 79%). The quality of the evidence was very low.There were no significant differences in other clinical outcomes including mortality and necrotising enterocolitis. For the outcomes of mortality and necrotising enterocolitis, the quality of the evidence was moderate. Long-term neurodevelopmental outcomes were not reported.
Authors' conclusions: Late administration of EPO reduces the use of one or more RBC transfusions, the number of RBC transfusions per infant (< 1 transfusion per infant) but not the total volume (mL/kg) of RBCs transfused per infant. Any donor exposure is likely not avoided as most studies included infants who had received RBC transfusions prior to trial entry. Late EPO does not significantly reduce or increase any clinically important adverse outcomes except for a trend in increased risk for ROP. Further research of the use of late EPO treatment, to prevent donor exposure, is not indicated. Research efforts should focus on limiting donor exposure during the first few days of life in sick neonates, when RBC requirements are most likely to be required and cannot be prevented by late EPO treatment. The use of satellite packs (dividing one unit of donor blood into many smaller aliquots) may reduce donor exposure.
Conflict of interest statement
None
Figures
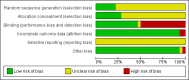
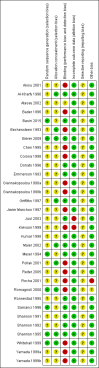

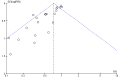



















Update of
-
Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants.Cochrane Database Syst Rev. 2014 Apr 23;(4):CD004868. doi: 10.1002/14651858.CD004868.pub4. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2019 Feb 15;2:CD004868. doi: 10.1002/14651858.CD004868.pub5. PMID: 24760628 Updated.
References
References to studies included in this review
-
- Al‐Kharfy T, Smyth JA, Wadsworth L, Krystal G, Fitzgerald C, Davis J, et al. Erythropoietin therapy in neonates at risk of having bronchopulmonary dysplasia and requiring multiple transfusions. Journal of Pediatrics 1996;129(1):89‐96. [PUBMED: 8757567] - PubMed
- Smyth JA, Ainsworth L, Krystal G, Wadsworth L. Effect of erythropoietin therapy on oxygen dependency in premature infants. Pediatric Research 2002;51(3):330 A.
-
- Atasay B, Gunlemez A, Akar N, Arsan S. Does early erythropoietin therapy decrease transfusions in anemia of prematurity?. Indian Journal of Pediatrics 2002;69(5):389‐91. [PUBMED: 12061670] - PubMed
-
- Bader D, Blondheim O, Jonas R, Admoni O, Abend‐Winge M, Reich D, et al. Decreased ferritin levels, despite iron supplementation, during erythropoietin therapy in anaemia of prematurity. Acta Paediatrica 1996;85(4):496‐501. [PUBMED: 8740313] - PubMed
-
- Basiri B, Shokouhi M, Pezeshki N, Torabian S. Beneficial erythropoetic effects of recombinant human erythropoietin in very low‐birth weight infants: a single‐center randomized double‐blinded placebo‐controlled trial. Journal of Clinical Neonatology 2015;4(2):87‐90. [DOI: 10.4103/2249-4847.154103] - DOI
References to studies excluded from this review
-
- Ahmadpour Kacho M, Zahed Pasha YA, Esmaili MR, Hajian K, Moradi SH. The effect of human recombinant erythropoietin on prevention of anemia of prematurity. Pediatric Research 2003;54(4):564.
-
- Amin AA, Alzahrani DM. Efficacy of erythropoietin in premature infants. Pediatric Research 2003;54(4):557. [PUBMED: 11938417] - PubMed
-
- Badiee Z, Pourmirzaiee MA, Kelishadi R, Naseri F. Recombinant human erythropoietin and blood transfusion in low‐birth weight preterm infants under restrictive transfusion guidelines. Saudi Medical Journal 2006;27(6):817‐20. [PUBMED: 16758042] - PubMed
-
- Bechensteen AG, Hågå P, Halvorsen S, Liestøl K, Lindemann R, Whitelaw A, et al. Effect of low and moderate doses of recombinant human erythropoietin on the haematological response in premature infants on high protein and iron intake. European Journal of Pediatrics 1997;156(1):56‐61. [PUBMED: 9007493] - PubMed
-
- Messer J, Haddad J, Donato L, Astruc D, Matis J. Early treatment of premature infants with recombinant human erythropoietin. Pediatrics 1993;92(4):519‐23. [PUBMED: 8414820] - PubMed
Additional references
-
- Brown MS, Phibbs RH, Gracia JF, Dallman PR. Postnatal changes in erythropoietin levels in untransfused premature infants. Journal of Pediatrics 1983;103(4):612‐7. [PUBMED: 6194281] - PubMed
-
- Cohen A, Manno C. Transfusion practices in infants receiving assisted ventilation. Clinics in Perinatology 1998;25(1):97‐111. [PUBMED: 9523077] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

