Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer
- PMID: 30776132
- PMCID: PMC6378927
- DOI: 10.1002/14651858.CD012873.pub2
Sequencing of anthracyclines and taxanes in neoadjuvant and adjuvant therapy for early breast cancer
Abstract
Background: Anthracyclines and taxanes are chemotherapeutic agents widely used in a sequential regimen in the adjuvant and neoadjuvant treatment of early breast cancer to reduce the risk of cancer recurrence. Standard practice is to administer anthracycline-based chemotherapy followed by a taxane. Anthracyclines tend to be administered first as they were established before taxanes for treatment of early breast cancer.
Objectives: To assess whether the sequence in which anthracyclines and taxanes are administered affects outcomes for people with early breast cancer receiving adjuvant or neoadjuvant therapy.
Search methods: We searched Cochrane Breast Cancer's Specialised Register, CENTRAL, MEDLINE, Embase, the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov on 1 February 2018.
Selection criteria: Randomised controlled trials comparing administering a taxane prior to an anthracycline with taxane following anthracycline to people with early breast cancer receiving chemotherapy. The studies needed to have reported on at least one of our outcomes of interest, which included overall survival, disease-free survival, pathological response, treatment adherence, toxicity and quality of life.
Data collection and analysis: Two review authors independently extracted data, assessed risk of bias and quality of the evidence. The primary outcome measure was overall survival. Secondary outcomes included disease-free survival, pathological response (in the neoadjuvant setting only), adverse events, treatment adherence and quality of life. For time-to-event outcomes of overall survival and disease-free survival, we derived hazard ratios (HRs) with 95% confidence intervals (CI) where possible. For dichotomous outcomes of pathological complete response, treatment adherence and adverse events, we reported the treatment effect as a risk ratio (RR) with 95% CI where possible. We used GRADE to assess the certainty of the evidence separately for the neoadjuvant and adjuvant settings.
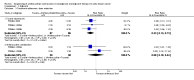
Main results: There were 1415 participants in five neoadjuvant studies and 280 participants in four adjuvant studies involving five treatment comparisons. Four of the five neoadjuvant studies collected data for the primary outcome (overall survival) and two studies had data available; one of the four adjuvant studies collected overall survival data.The neoadjuvant studies suggested that the administration of taxanes first probably resulted in little to no difference in overall survival (HR 0.80, 95% CI 0.60 to 1.08; 947 participants; 2 studies; moderate-certainty evidence) and disease-free survival (HR 0.84, 95% CI 0.65 to 1.09; 828 participants; 1 study; moderate-certainty evidence). Administration of taxanes first also resulted in little to no difference in pathological complete response (absence of cancer in the breast and axilla: RR 1.15, 95% CI 0.96 to 1.38; 1280 participants; 4 studies; high-certainty evidence). However, there appeared to be a trend in favour of taxanes first. Studies reported treatment adherence using a range of measures. Administration of taxanes first probably did not increase the likelihood of requiring dose reductions compared to administration of anthracyclines first (RR 0.81, 95% CI 0.59 to 1.11; 280 participants; 1 study; moderate-certainty evidence). There was probably little to no difference in the risk of grade 3/4 neutropenia (RR 1.25, 95% CI 0.86 to 1.82; 280 participants, 1 study; moderate-certainty evidence) or grade 3/4 neurotoxicity (RR 0.95, 95% CI 0.55 to 1.65; 1108 participants; 2 studies; low-certainty evidence) when taxanes were given first. There were no data on quality of life.Only one adjuvant study collected data on overall survival and disease-free survival but did not report data. Administration of taxanes first reduced the risk of grade 3/4 neutropenia (RR 0.62, 95% CI 0.40 to 0.97; 279 participants; 4 studies, 5 treatment comparisons; high-certainty evidence) and appeared to result in little to no difference in grade 3/4 neurotoxicity (RR 0.78, 95% CI 0.25 to 2.46; 162 participants; 3 studies; low-certainty evidence). There was probably little to no difference in the proportions experiencing dose delays when taxanes are given first compared to anthracyclines given first (RR 0.76, 95% CI 0.52 to 1.12; 238 participants; 3 studies, 4 treatment comparisons; moderate-certainty evidence). One study reported on quality of life and indicated that scores (using the Functional Assessment of Cancer Therapy - Breast Cancer (FACT-B) validated questionnaire) were similar in both groups though did not provide numerical data.
Authors' conclusions: In the neoadjuvant setting, there is high- to low-certainty evidence of equivalent outcomes for the sequence in which taxanes are delivered. In the adjuvant setting, none of the studies reported on overall survival or disease-free survival. In most institutions, standard practice would be to deliver anthracycline followed by taxane, and currently available data do not support a change in this practice. We wait for the full-text publication of a relevant neoadjuvant study for women with HER2-negative breast cancer for inclusion in an update of this review.
Conflict of interest statement
MZ: none known.
NW: none known.
MLW: none known.
DO'C: none known.
AG: none known.
Figures


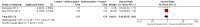
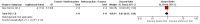











Update of
References
References to studies included in this review
Abe 2013 {published data only}
-
- Abe H, Mori T, Kawai Y, Cho H, Kubota Y, Umeda T, et al. Feasibility and toxicity of docetaxel before or after fluorouracil, epirubicin and cyclophosphamide as adjuvant chemotherapy for early breast cancer. International Journal of Clinical Oncology 2013;18:487‐91. - PubMed
ACOSOG Z1041 2013 {published data only}
-
- Buzdar A, Suman VJ, Meric‐Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al. ACOSOG Z1041 (Alliance): definitive analysis of randomized neoadjuvant trial comparing FEC followed by paclitaxel plus trastuzumab (FEC P+T) with paclitaxel plus trastuzumab followed by FEC plus trastuzumab (P+T FEC+T) in HER2+ operable breast cancer. Lancet 2013;14:1317‐25. - PMC - PubMed
-
- Buzdar A, Suman VJ, Meric‐Bernstam F, Leitch AM, Ellis MJ, Boughey JC, et al. ACOSOG Z1041 (Alliance): definitive analysis of randomized neoadjuvant trial comparing FEC followed by paclitaxel plus trastuzumab (FEC → P + T) with paclitaxel plus trastuzumab followed by FEC plus trastuzumab (P + T → FEC + T) in HER2+ operable breast cancer. Journal of Clinical Oncology 2013;15(Suppl):502.
AERO B03 2007 {published data only}
-
- Piedbois P, Serin D, Priou F, Laplaige P, Greget S, Angellier E, et al. Dose‐dense adjuvant chemotherapy in node‐positive breast cancer: docetaxel followed by epirubicin/cyclophosphamide (T/EC), or the reverse sequence (EC/T), every 2 weeks, versus docetaxel, epirubicin and cyclophosphamide (TEC) every 3 weeks. AERO B03 randomized phase II study. Annals of Oncology 2007;18:52‐7. - PubMed
Alamgeer 2014 {published data only}
-
- ACTRN12605000588695. Neoadjuvant chemotherapy with docetaxel and anthracycline based chemotherapy in patients with advanced breast cancer: evaluation of biological, clinical and imaging markers of tumour response. anzctr.org.au/trial/registration/ANTRN12605000588695 (first received 21 September 2005).
Miller 2005 {published data only}
-
- Miller KD, Soule SE, Calley C, Emerson RE, Hutchins GD, Kopecky K, et al. Randomized phase II trial of the anti‐angiogenic potential of doxorubicin and docetaxel; primary chemotherapy as Biomarker Discovery Laboratory. Breast Cancer Research and Treatment 2005;89:187‐97. - PubMed
Neo‐TAnGo 2014 {published data only}
-
- Earl HM, Vallier AL, Hiller L, Fenwick N, Young J, Iddawela M, et al. Effects of the addition of gemcitabine, and paclitaxel‐first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high‐risk early breast cancer (Neo‐tAnGo): an open‐label, 2×2 factorial randomised phase 3 trial. Lancet Oncology 2014;15:201‐12. - PubMed
-
- NCT00070278. Neoadjuvant epirubicin, cyclophosphamide, and paclitaxel with or without gemcitabine in treating women who are undergoing surgery for early breast cancer. clinicaltrials.gov/show/NCT00070278 (first received 7 October 2003).
Puhalla 2008 {published data only}
-
- NCT00201708. Dose‐dense docetaxel before or after doxorubicin/cyclophosphamide in axillary node‐positive breast cancer. clinicaltrials.gov/show/NCT00201708 (first received 20 September 2005).
-
- Puhalla S, Mrozek E, Young D, Ottman S, McVey A, Kendra K, et al. Randomized phase II adjuvant trial of dose‐dense docetaxel before or after doxorubicin plus cyclophosphamide in axillary node‐positive breast cancer. Journal of Clinical Oncology 2008;26(10):1691‐7. - PubMed
Stearns 2003 {published data only}
-
- Stearns V, Singh B, Tsangaris T, Crawford JG, Novielli A, Ellis MJ, et al. 2003. A prospective randomized pilot study to evaluate predictors of response in serial core biopsies to single agent neoadjuvant doxorubicin or paclitaxel for patients with locally advanced breast cancer 2003;9:124‐33. - PubMed
Wildiers 2009a {published data only}
-
- Wildiers H, Dirix L, Neven P, Prové A, Clement P, Amant F, et al. Chemotherapy dose delays and dose reductions in breast cancer patients receiving dose‐dense FEC and docetaxel – results of a randomized, open‐label phase II study. 30th Annual San Antonio Breast Cancer Symposium; 2007 Dec 13‐16; San Antonio (TX). 2007; Vol. S150:3072.
-
- Wildiers H, Dirix L, Neven P, Prové A, Clement P, Squifflet P, et al. Delivery of adjuvant sequential dose‐dense FEC‐Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Research and Treatment 2009;114:103‐12. - PubMed
Wildiers 2009b {published data only}
-
- Wildiers H, Dirix L, Neven P, Prové A, Clement P, Amant F, et al. Chemotherapy dose delays and dose reductions in breast cancer patients receiving dose‐dense FEC and docetaxel – results of a randomized, open‐label phase II study. 30th Annual San Antonio Breast Cancer Symposium; 2007 Dec 13‐16; San Antonio (TX). 2007; Vol. S150:3072.
-
- Wildiers H, Dirix L, Neven P, Prové A, Clement P, Squifflet P, et al. Delivery of adjuvant sequential dose‐dense FEC‐Doc to patients with breast cancer is feasible, but dose reductions and toxicity are dependent on treatment sequence. Breast Cancer Research and Treatment 2009;114:103‐12. - PubMed
References to studies excluded from this review
Akashi‐Tanaka 2017 {published data only}
-
- Akashi‐Tanaka S, Tanino Y, Yamamoto Y, Nishimiya H, Yamamoto‐ibusuki M, Iwase H, et al. BRCAness and prognosis of triple‐negative breast cancer patients treated with neoadjuvant chemotherapy. Journal of Clinical Oncology 2017;35(15 Suppl 1):e12111.
Albain 2012 {published data only}
Anonymous 2001 {published data only}
-
- Anonymous. Epirubicin and cyclophosphamide (EC) vs docetaxel (d) followed by EC in adjuvant (ADJ) treatment of node positive (pN1) breast cancer (BC) – a multicenter randomized phase III study. Proceedings of the American Society of Clinical Oncology 2001;20(Part 2):22b.
Buzdar 2004 {published data only}
-
- Buzdar A, Hunt K, Smith T, Francis D, Ewer M, Booser D, et al. Significantly higher pathological complete remission (PCR) rate following neoadjuvant therapy with trastuzumab (H), paclitaxel (P), and anthracycline‐containing chemotherapy (CT): initial results of a randomized trial in operable breast cancer (BC) with HER/2 positive disease. Journal of Clinical Oncology 2004;7:520.
Cardoso 2001 {published data only}
-
- Cardoso F, Ferreira FA, Crown J, Dolci S, Paesmans M, Riva A, et al. Doxorubicin followed by docetaxel versus docetaxel followed by doxorubicin in the adjuvant treatment of node positive breast cancer: results of a feasibility study. Anticancer Research 2001;21(1b):789‐95. - PubMed
Cresta 2001 {published data only}
-
- Cresta S, Graselli G, Martoni A, Lelli G, Mansutti M, Capri G, et al. A randomized phase II study of alternating (AA) vs sequential (SS) vs the combination (CC) of doxorubicin (A) and docetaxel (T) as 1st line CT in MBC PTS. Proceedings of the American Society of Clinical Oncology 2001;20(Pt 1):48a.
Earl 2003 {published data only}
-
- Earl H. Phase I/II randomized study of neoadjuvant sequential epirubicin and docetaxel in women with poor‐risk early breast cancer. Physician Data Query 2003.
Fabiano 2002 {published data only}
-
- Fabiano A, Filho F, Crown J, Cardoso F, Nogaret JM, Duffy K, et al. 3‐Year results of docetaxel‐based sequential and combination regimens in the adjuvant therapy of node‐positive breast cancer: a pilot study. Anticancer Research 2002; Vol. 22, issue 4:2471‐6. - PubMed
Focan 2005 {published data only}
-
- Focan C, Graas M, Beauduin M, Canon J, Salmon J, Jerusalem G, et al. Sequential administration of epirubicin and paclitaxel for advanced breast cancer. A phase I randomised trial. Anticancer Research 2005;25(2b):1211‐7. - PubMed
Guarneri 2010 {published data only}
-
- Guarneri V, Frassoldati A, Gebbia V, Bisagni G, Cavanna L, Donadio M. 9 weeks vs 1 year adjuvant trastuzumab in combination with chemotherapy: preliminary cardiac safety data of the phase III multicentric Italian study short‐HER. Cancer Research 2010;70(24 Suppl):P5‐12‐05.
Skarlos 2012 {published data only}
-
- Skarlos P, Christodoulou C, Kalogeras KT, Eleftheraki AG, Bobos M, Batistatou A, et al. Triple‐negative phenotype is of adverse prognostic value in patients treated with dose‐dense sequential adjuvant chemotherapy: a translational research analysis in the context of a Hellenic Cooperative Oncology Group (HeCOG) randomized phase III trial. Cancer Chemotherapy and Pharmacology 2012;69(2):533‐46. - PubMed
SWOG S0800 {published data only}
-
- Nahleh ZA, Barlow WE, Hayes DF, Schott AF, Gralow JR, Sikov WM, et al. SWOG S0800 (NCI CDR0000636131): addition of bevacizumab to neoadjuvant nab‐paclitaxel with dose‐dense doxorubicin and cyclophosphamide improves pathologic complete response (pCR) rates in inflammatory or locally advanced breast cancer. Breast Cancer Research and Treatment 2016;158(3):485‐95. - PMC - PubMed
Thomas 2017 {published data only}
-
- Thomas J, Provenzano E, Hiller L, Dunn J, Blenkinsop C, Grybowicz L, et al. Central pathology review with two‐stage quality assurance for pathological response after neoadjuvant chemotherapy in the ARTemis Trial. Modern Pathology 2017;30(8):1069‐77. - PubMed
Wildiers 2006 {published data only}
-
- Wildiers H. A randomized phase II trial exploring feasibility of densification and optimal sequencing of postoperative adjuvant fluorouracil, epirubicin plus cyclophosphamide (FEC) and docetaxel chemotherapy in patients with high risk primary operable breast cancer. Physician Data Query 2006.
References to studies awaiting assessment
Masuda 2012 {published data only}
-
- Masuda N, Sato N, Higaki K, Kashiwaba M, Matsunami N, Takano T, et al. A prospective multicenter randomized phase II neo‐adjuvant study of 5‐fluorouracil epirubicin and cyclophosphamide (FEC) followed by docetaxel cyclophosphamide and trastuzumab (TCH) versus TCH followed by FEC versus TCH alone in patients (pts) with operable HER2 positive breast cancer: JBCRG‐10 study. San Antonio Breast Cancer Symposium; 2012 Dec 4‐8; San Antonio (TX). 2012:P1‐14‐01.
NeoSAMBA {published data only}
-
- NCT012700373. NeoSAMBA neoadjuvant: does the sequence of anthracycline and taxane matter: before or after?. clinical.tirals.gov/show/NCT01270373 (first received 5 January 2011).
Taghian 2005 {published data only}
-
- Corben AD, Abi‐Raad R, Popa I, Teo CH, Macklin EA, Koerner FC, et al. Pathologic response and long‐term follow‐up in breast cancer patients treated with neoadjuvant chemotherapy. Archives of Pathology & Laboratory Medicine 2013;137:1074‐82. - PubMed
-
- Taghian AG, Abi‐Raad R, Assaad SI, Casty A, Ancukiewicz M, Yeh E, et al. Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. Journal of Clinical Oncology 2005;23(9):1951‐61. - PubMed
References to ongoing studies
UMIN000003283 {published data only}
-
- UMIN000003283. A randomized study of docetaxel + cyclophosphamide (TC), 5‐fluorouracil + epirubicin + cyclophosphamide (FEC)‐TC and TC‐FEC as preoperative chemotherapy for hormone receptor positive and HER2 negative primary breast cancer JBCRG‐09. https://upload.umin.ac.jp/cgi‐open‐bin/ctr/ctr.cgi?function=brows&ac... Date first received: 3 March 2010.
Additional references
AJCC 2010
-
- American Joint Committee on Cancer. Breast. In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A editor(s). AJCC Cancer Staging Manual. 7th Edition. New York (NY): Springer, 2010:347‐69.
Alvarez 2010
-
- Alvarez RH, Bianchini G, Hsu L, Cristofanilli M, Esteva FJ, Pusztai L, et al. Clinical outcome of two sequences of administering paclitaxel (P) and anthracyclines (A) as primary systemic therapy (PST) and adjuvant chemotherapy (ACT) in breast cancer (BC) patients: a retrospective analysis from the M.D. Anderson Cancer Centre. Cancer Research 2010;70(24 Suppl):384s (abstract P5‐10‐02).
Bines 2014
-
- Bines J, Earl H, Buzaid AC, Saad ED. Anthracyclines and taxanes in the neo/adjuvant treatment of breast cancer: does the sequence matter?. Annals of Oncology 2014;25(6):1079‐85. - PubMed
Cochran 1954
-
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10(1):101‐29.
Cossetti 2015
-
- Cossetti RJD, Tyldesley SK, Speers CH, Zheng Y, Gelmon KA. Comparison of breast cancer recurrence and outcome patterns between patients treated from 1986 to 1992 and from 2004 to 2008. Journal of Clinical Oncology 2015;33(1):65‐73. - PubMed
Covidence
-
- Covidence systematic review software. Veritas Health Innovation, Melbourne, Available at www.covidence.org.
Deeks 2011
-
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Endnote [Computer program]
-
- Thomson Reuters. Endnote X7. Thomson Reuters, 2014.
Ferlay 2015
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer 2015;136(5):E359‐86. - PubMed
GRADEpro GDT
-
- GRADEproGDT. McMaster University (developed by Evidence Prime), Hamilton (ON). Available at gradepro.org.
Guyatt 2011
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence – imprecision. Journal of Clinical Epidemiology 2011;64:1283‐93. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jones 2006
-
- Jones SE, Savin MA, Holmes FA, O'Shaughnessy JA, Blum JL, Vukelja S, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. Journal of Clinical Oncology 2006;24(34):5381‐7. - PubMed
Levine 1998
-
- Levine MN, Bramwell VH, Pritchard KI, Norris BD, Shepherd LE, Abu‐Zahra H, et al. Randomized trial of intensive cyclophosphamide, epirubicin, and fluorouracil chemotherapy compared with cyclophosphamide, methotrexate, and fluorouracil in premenopausal women with node‐positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. Journal of Clinical Oncology 1998;16(8):2651‐8. - PubMed
Mantel 1959
-
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719‐48. - PubMed
MD Anderson Cancer Center
-
- MD Anderson Cancer Center. Residual Cancer Burden calculator and associated documents (guide for measuring cancer cellularity, examples of gross & microscopic evaluation, pathology protocol for macroscopic and microscopic assessment of RCB). www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3 (accessed 12 April 2017).
Moher 2009
NCI‐CTCAE
-
- U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE), Version 4.011. evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf (accessed 07 November 2017).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

