Simvastatin promotes restoration of chondrocyte morphology and phenotype
- PMID: 30776329
- PMCID: PMC6450767
- DOI: 10.1016/j.abb.2019.01.038
Simvastatin promotes restoration of chondrocyte morphology and phenotype
Abstract
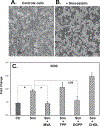
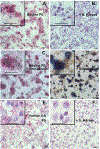
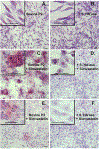
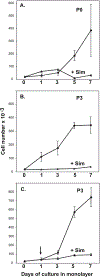
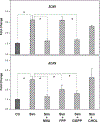
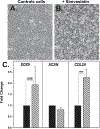
In this study we examined whether the action of simvastatin affects re-differentiation of passaged chondrocytes and if so, whether this was mediated via changes in cholesterol or cholesterol intermediates. Bovine articular chondrocytes, of varying passage number, human knee chondrocytes and rat chondrosarcoma chondrocytes were treated with simvastatin and examined for changes in mRNA and protein expression of markers of the chondrocyte phenotype as well as changes in cell shape, proliferation and proteoglycan production. In all three models, while still in monolayer culture, simvastatin treatment alone promoted changes in phenotype and morphology indicative of re-differentiation most prominent being an increase in SOX9 mRNA and protein expression. In passaged bovine chondrocytes, simvastatin stimulated the expression of SOX9, ACAN, BMP2 and inhibited the expression of COL1 and α-smooth muscle actin. Co-treatment of chondrocytes with simvastatin plus exogenous cholesterol-conditions that had previously reversed the inhibition on CD44 shedding, did not alter the effects of simvastatin on re-differentiation. However, the co-treatment of chondrocytes with simvastatin together with other pathway intermediates, mevalonate, geranylgeranylpyrophosphate and to a lesser extent, farnesylpyrophosphate, blocked the pro-differentiation effects of simvastatin. Treatment with simvastatin stimulated expression of SOX9 and COL2a and enhanced SOX9 protein in human OA chondrocytes. The co-treatment of OA chondrocytes with mevalonate or geranylgeranylpyrophosphate, but not cholesterol, blocked the simvastatin effects. These results lead us to conclude that the blocking of critical protein prenylation events is required for the positive effects of simvastatin on the re-differentiation of chondrocytes.
Keywords: Aggrecan; Chondrocyte; Osteoarthritis; Prenylation; SOX9; Simvastatin.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Interplay between cytoskeletal polymerization and the chondrogenic phenotype in chondrocytes passaged in monolayer culture.J Anat. 2017 Feb;230(2):234-248. doi: 10.1111/joa.12554. Epub 2016 Nov 3. J Anat. 2017. PMID: 27807861 Free PMC article.
-
Simvastatin induces differentiation of rabbit articular chondrocytes via the ERK-1/2 and p38 kinase pathways.Exp Cell Res. 2016 Aug 15;346(2):198-205. doi: 10.1016/j.yexcr.2016.07.025. Epub 2016 Jul 27. Exp Cell Res. 2016. PMID: 27475840
-
Retroviral transduction with SOX9 enhances re-expression of the chondrocyte phenotype in passaged osteoarthritic human articular chondrocytes.Osteoarthritis Cartilage. 2005 Jan;13(1):80-9. doi: 10.1016/j.joca.2004.10.011. Osteoarthritis Cartilage. 2005. PMID: 15639641
-
Simvastatin inhibits CD44 fragmentation in chondrocytes.Arch Biochem Biophys. 2016 Aug 15;604:1-10. doi: 10.1016/j.abb.2016.05.019. Epub 2016 May 28. Arch Biochem Biophys. 2016. PMID: 27242325
-
Transforming growth factor alpha suppression of articular chondrocyte phenotype and Sox9 expression in a rat model of osteoarthritis.Arthritis Rheum. 2007 Nov;56(11):3693-705. doi: 10.1002/art.22968. Arthritis Rheum. 2007. PMID: 17968906
Cited by
-
Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms.Bone Res. 2023 Dec 6;11(1):63. doi: 10.1038/s41413-023-00301-9. Bone Res. 2023. PMID: 38052778 Free PMC article. Review.
-
Pharmaceutical therapeutics for articular regeneration and restoration: state-of-the-art technology for screening small molecular drugs.Cell Mol Life Sci. 2021 Dec;78(24):8127-8155. doi: 10.1007/s00018-021-03983-8. Epub 2021 Nov 16. Cell Mol Life Sci. 2021. PMID: 34783870 Free PMC article. Review.
-
Restoration of the Phenotype of Dedifferentiated Rabbit Chondrocytes by Sesquiterpene Farnesol.Pharmaceutics. 2022 Jan 13;14(1):186. doi: 10.3390/pharmaceutics14010186. Pharmaceutics. 2022. PMID: 35057081 Free PMC article.
-
Hyaluronan synthase 2 (HAS2) overexpression diminishes the procatabolic activity of chondrocytes by a mechanism independent of extracellular hyaluronan.J Biol Chem. 2019 Sep 13;294(37):13562-13579. doi: 10.1074/jbc.RA119.008567. Epub 2019 Jul 3. J Biol Chem. 2019. PMID: 31270213 Free PMC article.
-
Fluvastatin promotes chondrogenic differentiation of adipose-derived mesenchymal stem cells by inducing bone morphogenetic protein 2.BMC Pharmacol Toxicol. 2022 Aug 9;23(1):61. doi: 10.1186/s40360-022-00600-7. BMC Pharmacol Toxicol. 2022. PMID: 35945639 Free PMC article.
References
-
- Stokes DG, Liu G, Coimbra IB, Piera-Velazquez S, Crowl RM, Jimenez SA, Assessment of the gene expression profile of differentiated and dedifferentiated human fetal chondrocytes by microarray analysis, Arthritis Rheum 46 (2002) 404–419. - PubMed
-
- Cha BH, Kim JH, Kang SW, Do HJ, Jang JW, Choi YR, et al., Cartilage tissue formation from dedifferentiated chondrocytes by codelivery of BMP-2 and SOX-9 genes encoding bicistronic vector, Cell Transplant. 22 (2013) 1519–1528. - PubMed
-
- Parreno J, Bianchi VJ, Sermer C, Regmi SC, Backstein D, Schmidt TA, et al., Adherent agarose mold cultures: An in vitro platform for multi-factorial assessment of passaged chondrocyte redifferentiation, J. Orthop. Res 36 (2018) 2392–2405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

