Autocrine production of reproductive axis neuropeptides affects proliferation of canine osteosarcoma in vitro
- PMID: 30777054
- PMCID: PMC6379937
- DOI: 10.1186/s12885-019-5363-4
Autocrine production of reproductive axis neuropeptides affects proliferation of canine osteosarcoma in vitro
Abstract
Background: Osteosarcoma strikes hundreds of people each year, of both advanced and younger ages, and is often terminal. Like many tumor types, these bone tumors will frequently undergo a neuroendocrine transition, utilizing autocrine and/or paracrine hormones as growth factors and/or promoters of angiogenesis to facilitate progression and metastasis. While many of these factors and their actions on tumor growth are characterized, some tumor-derived neuropeptides remain unexplored.
Methods: Using validated canine osteosarcoma cell lines in vitro, as well as cells derived from spontaneous tumors in dogs, we explored the autocrine production of two neuropeptides typically found in the hypothalamus, and most closely associated with reproduction: gonadotropin-releasing hormone (GnRH) and kisspeptin (Kiss-1). We evaluated gene expression and protein secretion of these hormones using quantitative RT-PCR and a sensitive radioimmunoassay, and explored changes in cell proliferation determined by MTS cell viability assays.
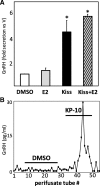
Results: Our current studies reveal that several canine osteosarcoma cell lines (COS, POS, HMPOS, D17, C4) synthesize and secrete GnRH and express the GnRH receptor, while COS and POS also express kiss1 and its cognate receptor. We have further found that GnRH and kisspeptin, exogenously applied to these tumor cells, exert significant effects on both gene expression and proliferation. Of particular interest, kisspeptin exposure stimulated GnRH secretion from COS, similarly to the functional relationship observed within the neuroendocrine reproductive axis. Additionally, GnRH and kisspeptin treatment both increased COS proliferation, which additionally manifested in increased expression of the bone remodeling ligand rankl within these cells. These effects were blocked by treatment with a specific GnRH receptor inhibitor. Both neuropeptides were found to increase expression of the specific serotonin (5HT) receptor htr2a, the activation of which has previously been associated with cellular proliferation, suggesting that production of these factors by osteosarcoma cells may act to sensitize tumors to circulating 5HT of local and/or enteric origin.
Conclusions: Here we report that kisspeptin and GnRH act as autocrine growth factors in canine osteosarcoma cells in vitro, modulating RANKL and serotonin receptor expression in a manner consistent with pro-proliferative effects. Pharmacological targeting of these hormones may represent new avenues of osteosarcoma treatment.
Keywords: Autocrine; GnRH; Kisspeptin; Osteosarcoma; Proliferation.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





References
-
- Anderson ME. Update on survival in osteosarcoma. Orthop Clin North Am 2016;47(1):283–292. doi: 10.1016/j.ocl.2015.08.022. PubMed PMID: 26614941. - PubMed
-
- Mueller F, Fuchs B, Kaser-Hotz B. Comparative biology of human and canine osteosarcoma. Anticancer Res. 2007;27(1A):155–164. - PubMed
-
- Barroga E, Kadosawa T, Okumura M. Establishment and characterization of the growth and pulmonary metastasis of a highly lung metastasizing cell line from canine osteosarcoma in nude mice. J Vet Med Sci 1999;61:361–7. PubMed PMID: doi:10.1292/jvms.61.361. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

