Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial
- PMID: 30777118
- PMCID: PMC6379931
- DOI: 10.1186/s13063-019-3184-z
Impact of two oral doses of 100,000 IU of vitamin D3 in preschoolers with viral-induced asthma: a pilot randomised controlled trial
Abstract
Background: New evidence supports the use of supplemental vitamin D in the prevention of exacerbation of asthma; however, the optimal posology to sufficiently raise serum levels while maximising adherence is unclear. The objective was to ascertain the efficacy of high-dose vitamin D3 in increasing serum vitamin D in preschoolers with asthma and provide preliminary data on safety and efficacy outcomes.
Methods: We conducted a 7-month, triple-blind, randomised, placebo-controlled, pilot trial of children aged 1-5 years with viral-induced asthma. Participants were allocated to receive two oral doses of 100,000 IU vitamin D3 (intervention) or identical placebo (control) 3.5 months apart, once in the fall and once in the winter. Serum 25-hydroxyvitamin D (25OHD) was measured by tandem mass spectrometry at baseline, 10 days, 3.5 months, 3.5 months + 10 days, and 7 months. The main outcome was the change in serum 25OHD from baseline (Δ25OHD) over time and at 3.5 and 7 months; other outcomes included the proportion of children with 25OHD ≥ 75 nmol/L, safety, and adverse event rates.
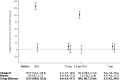
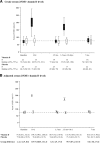
Results: Children (N = 47) were randomised (intervention, 23; control, 24) in the fall. There was a significant adjusted group difference in the Δ25OHD (95% confidence interval) of 57.8 (47.3, 68.4) nmol/L, p < 0.0001), with a time (p < 0.0001) and group*time interaction effect (p < 0.0001), in favour of the intervention. A significant group difference in the Δ25OHD was observed 10 days after the first (119.3 [105.8, 132.9] nmol/L) and second (100.1 [85.7, 114.6] nmol/L) bolus; it did not reach statistical significance at 3.5 and 7 months. At 3.5 and 7 months, respectively, 63% and 56% of the intervention group were vitamin D sufficient (≥ 75 nmol/L) compared to 39% and 36% of the control group. Hypercalciuria, all without hypercalcaemia, was observed in 8.7% of intervention and 10.3% of control samples at any time point. Exacerbations requiring rescue oral corticosteroids, which appear as a promising primary outcome, occurred at a rate of 0.87/child.
Conclusion: Two oral boluses of 100,000 IU vitamin D3,once in the fall and once in the winter, rapidly, safely, and significantly raises overall serum vitamin D metabolites. However, it is sufficient to maintain 25OHD ≥ 75 nmol/L throughout 7 months in only slightly more than half of participants.
Trial registration: ClinicalTrials.gov, NCT02197702 (23 072014). Registered on 23 July 2014.
Keywords: Asthma; Child; Cholecalciferol; Paediatric; Pilot study; Randomised controlled trial; Viral-induced; Vitamin D.
Conflict of interest statement
Ethics approval and consent to participate
Parents provided written informed consent for their child’s study participation and for the release of medical and pharmacy data. The Institutional Research Ethics Board of the Sainte-Justine University Health Centre (#2015–786, 4004) and Health Canada approved the study (#187438).
Consent for publication
Not applicable.
Competing interests
Euro-Pharm (Montreal, Canada) elaborated and donated the study drug formulation, but had no input in the study design, conduct, analysis, and writing of the study. The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Moorman JE, Akinbami LJ, Bailey CM, Zahran HS, King ME, Johnson CA, Liu X. National surveillance of asthma: United States, 2001-2010. Vital Health Stat. 2012;3(35):1–58. - PubMed
-
- Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, Hopman WM, Lam M, Sears MR, Szpiro K, et al. The Ontario asthma regional variation study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129(4):909–917. doi: 10.1378/chest.129.4.909. - DOI - PubMed

