Culture of and experiments with sea urchin embryo primary mesenchyme cells
- PMID: 30777181
- PMCID: PMC8273911
- DOI: 10.1016/bs.mcb.2019.01.002
Culture of and experiments with sea urchin embryo primary mesenchyme cells
Abstract
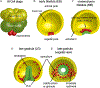
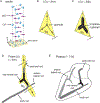
Skeletogenesis in the sea urchin embryo gives rise to a pair of intricate endoskeletal spicules. Deposition of these skeletal elements in the early larva is the outcome of a morphogenetic program that begins with maternal inputs in the early zygote and results in the specification of the large micromere-primary mesenchyme cell (PMC) lineage. PMCs are of considerable interest as a model system, not only to dissect the mechanism of specific developmental processes, but also to investigate their evolution and the unrivaled level of control over the formation of a graded, mechanically robust, yet single crystalline biomineral. The ability to study gene regulatory circuits, cellular behavior, signaling pathways, and molecular players involved in biomineralization is significantly boosted by the high level of autonomy of PMCs. In fact, in the presence of horse serum, micromeres differentiate into PMCs and produce spicules in vitro, separated from the embryonic milieu. PMC culture eliminates indirect effects that can complicate the interpretation of experiments in vivo, offers superior spatiotemporal control, enables PMC-specific readouts, and is compatible with most imaging and characterization techniques. In this chapter, we provide an updated protocol, based on the pioneering work by Okazaki and Wilt, for the isolation of micromeres and subsequent culture of PMCs, as well as protocols for fixation and staining for fluorescent microscopy, preparation of cell cultures for electron microscopy, and the isolation of RNA.
Keywords: Cell culture; Development; Electron microscopy; Fluorescence microscopy; Primary mesenchyme cells; Sea urchin embryo; Skeletogenesis; Spiculogenesis; Transcriptomics.
© 2019 Elsevier Inc. All rights reserved.
Figures











References
-
- Addadi L, Raz S, & Weiner S (2003). Taking advantage of disorder: Amorphous calcium carbonate and its roles in biomineralization. Advanced Materials, 15, 959–970.
-
- Adomako-Ankomah A, & Ettensohn CA (2013). Growth factor-mediated mesodermal cell guidance and skeletogenesis during sea urchin gastrulation. Development, 140, 4214–4225. - PubMed
-
- Amemiya S (1989). Development of the basal lamina and its role in migration and pattern-formation of primary mesenchyme cells in sea-urchin embryos. Development, Growth & Differentiation, 31, 131–145. - PubMed
-
- Ameye L, Compere P, Dille J, & Dubois P (1998). Ultrastructure and cytochemistry of the early calcification site and of its mineralization organic matrix in Paracentrotus lividus (Echinodermata: Echinoidea). Histochemistry and Cell Biology, 110, 285–294. - PubMed
-
- Ameye L, Hermann R, & Dubois P (2000). Ultrastructure of sea urchin calcified tissues after high-pressure freezing and freeze substitution. Journal of Structural Biology, 131, 116–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

