Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain
- PMID: 30777863
- PMCID: PMC6398449
- DOI: 10.1242/dev.156059
Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain
Abstract
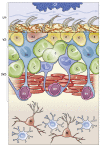
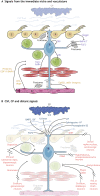
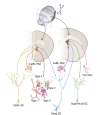
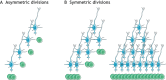
In the adult rodent brain, neural stem cells (NSCs) persist in the ventricular-subventricular zone (V-SVZ) and the subgranular zone (SGZ), which are specialized niches in which young neurons for the olfactory bulb (OB) and hippocampus, respectively, are generated. Recent studies have significantly modified earlier views on the mechanisms of NSC self-renewal and neurogenesis in the adult brain. Here, we discuss the molecular control, heterogeneity, regional specification and cell division modes of V-SVZ NSCs, and draw comparisons with NSCs in the SGZ. We highlight how V-SVZ NSCs are regulated by local signals from their immediate neighbors, as well as by neurotransmitters and factors that are secreted by distant neurons, the choroid plexus and vasculature. We also review recent advances in single cell RNA analyses that reveal the complexity of adult neurogenesis. These findings set the stage for a better understanding of adult neurogenesis, a process that one day may inspire new approaches to brain repair.
Keywords: Differentiation; Neural stem cells; Neurogenesis; Self-renewal; Stem cell heterogeneity; Transcriptomics.
© 2019. Published by The Company of Biologists Ltd.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

