BCL6 Evolved to Enable Stress Tolerance in Vertebrates and Is Broadly Required by Cancer Cells to Adapt to Stress
- PMID: 30777872
- PMCID: PMC6497559
- DOI: 10.1158/2159-8290.CD-17-1444
BCL6 Evolved to Enable Stress Tolerance in Vertebrates and Is Broadly Required by Cancer Cells to Adapt to Stress
Abstract
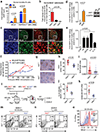
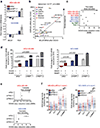
Several lines of evidence link the canonical oncogene BCL6 to stress response. Here we demonstrate that BCL6 evolved in vertebrates as a component of the HSF1-driven stress response, which has been co-opted by the immune system to support germinal center formation and may have been decisive in the convergent evolution of humoral immunity in jawless and jawed vertebrates. We find that the highly conserved BTB corepressor binding site of BCL6 mediates stress adaptation across vertebrates. We demonstrate that pan-cancer cells hijack this stress tolerance mechanism to aberrantly express BCL6. Targeting the BCL6 BTB domain in cancer cells induces apoptosis and increases susceptibility to repeated doses of cytotoxic therapy. The chemosensitization effect upon BCL6 BTB inhibition is dependent on the derepression of TOX, implicating modulation of DNA repair as a downstream mechanism. Collectively, these data suggest a form of adaptive nononcogene addiction rooted in the natural selection of BCL6 during vertebrate evolution. SIGNIFICANCE: We demonstrate that HSF1 drives BCL6 expression to enable stress tolerance in vertebrates. We identify an HSF1-BCL6-TOX stress axis that is required by cancer cells to tolerate exposure to cytotoxic agents and points toward BCL6-targeted therapy as a way to more effectively kill a wide variety of solid tumors.This article is highlighted in the In This Issue feature, p. 565.
©2019 American Association for Cancer Research.
Conflict of interest statement
Disclosure of potential conflicts of interest:
A.M. reports receiving commercial research grants from Janssen. L.C. receives research funds from Celgene and Karyopharm. No potential conflicts of interest were disclosed by the other authors.
Figures





References
-
- Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM. Control of Inflammation, Cytokine Expression, and Germinal Center Formation by BCL-6. Science 1997;276(5312):589–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

