Adenosine deaminase-1 delineates human follicular helper T cell function and is altered with HIV
- PMID: 30778076
- PMCID: PMC6379489
- DOI: 10.1038/s41467-019-08801-1
Adenosine deaminase-1 delineates human follicular helper T cell function and is altered with HIV
Abstract
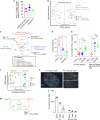
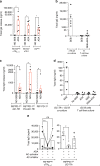
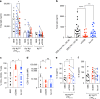
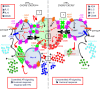
Follicular helper T cells (Tfh) play critical roles instructing, and initiating T-cell dependent antibody responses. The underlying mechanisms that enhance their function is therefore critical for vaccine development. Here we apply gene array analysis identifying adenosine deaminase (ADA) as a key molecule that delineates a human Tfh helper program in proliferating circulating Tfh (cTfh) cells and Germinal Centers Tfh (GC-Tfh). ADA-1 expression and enzymatic activity are increased in efficient cTfh2-17/GC-Tfh cells. Exogenous ADA-1 enhances less efficient cTfh1 and pro-follicular Tfh PD-1+ CXCR5+ cells to provide B cell help, while pharmacological inhibition of ADA-1 activity impedes cTfh2-17/GC-Tfh function and diminished antibody response. Mechanistically, ADA-1 controls the Tfh program by influencing IL6/IL-2 production, controlling CD26 extracellular expression and could balance signals through adenosine receptors. Interestingly, dysfunctional Tfh from HIV infected-individual fail to regulate the ADA pathway. Thus, ADA-1 regulates human Tfh and represents a potential target for development of vaccine strategy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Hershfield, M. in GeneReviews (eds Pagon, R. A. et al) Ch: Adenosine deaminase deficiency (University of Washington, 1993). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

