Persistent autonomic dysfunction and bladder sensitivity in primary dysmenorrhea
- PMID: 30778114
- PMCID: PMC6379479
- DOI: 10.1038/s41598-019-38545-3
Persistent autonomic dysfunction and bladder sensitivity in primary dysmenorrhea
Abstract
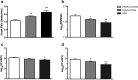
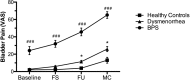
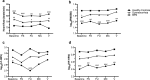
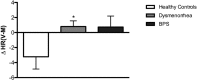
Menstrual pain, also known as dysmenorrhea, is a leading risk factor for bladder pain syndrome (BPS). A better understanding of the mechanisms that predispose dysmenorrheic women to BPS is needed to develop prophylactic strategies. Abnormal autonomic regulation, a key factor implicated in BPS and chronic pain, has not been adequately characterized in women with dysmenorrhea. Thus, we examined heart rate variability (HRV) in healthy (n = 34), dysmenorrheic (n = 103), and BPS participants (n = 23) in their luteal phase across a bladder-filling task. Both dysmenorrheic and BPS participants reported increased bladder pain sensitivity when compared to controls (p's < 0.001). Similarly, dysmenorrheic and BPS participants had increased heart rate (p's < 0.01), increased diastolic blood pressure (p's < 0.01), and reduced HRV (p's < 0.05) when compared to controls. Dysmenorrheic participants also exhibited little change in heart rate between maximum bladder capacity and after micturition when compared to controls (p = 0.013). Our findings demonstrate menstrual pain's association with abnormal autonomic activity and bladder sensitivity, even two weeks after menses. Our findings of autonomic dysfunction in both early episodic and chronic visceral pain states points to an urgent need to elucidate the development of such imbalance, perhaps beginning in adolescence.
Conflict of interest statement
Dr. Frank Tu has consulted for AbbVie Inc, served on their Speaker Bureau, and received compensation. The remaining authors declare no competing interests.
Figures
Similar articles
-
Clinical profile of comorbid dysmenorrhea and bladder sensitivity: a cross-sectional analysis.Am J Obstet Gynecol. 2020 Jun;222(6):594.e1-594.e11. doi: 10.1016/j.ajog.2019.12.010. Epub 2019 Dec 20. Am J Obstet Gynecol. 2020. PMID: 31870730 Free PMC article.
-
Abdominal skeletal muscle activity precedes spontaneous menstrual cramping pain in primary dysmenorrhea.Am J Obstet Gynecol. 2018 Jul;219(1):91.e1-91.e7. doi: 10.1016/j.ajog.2018.04.050. Epub 2018 May 5. Am J Obstet Gynecol. 2018. PMID: 29733841 Free PMC article.
-
Identification of experimental bladder sensitivity among dysmenorrhea sufferers.Am J Obstet Gynecol. 2018 Jul;219(1):84.e1-84.e8. doi: 10.1016/j.ajog.2018.04.030. Epub 2018 Apr 25. Am J Obstet Gynecol. 2018. PMID: 29704486 Free PMC article.
-
What we know about primary dysmenorrhea today: a critical review.Hum Reprod Update. 2015 Nov-Dec;21(6):762-78. doi: 10.1093/humupd/dmv039. Epub 2015 Sep 7. Hum Reprod Update. 2015. PMID: 26346058 Review.
-
Eicosanoids in primary dysmenorrhea, endometriosis and menstrual migraine.Gynecol Endocrinol. 1989;3(1):71-94. doi: 10.3109/09513598909152454. Gynecol Endocrinol. 1989. PMID: 2658474 Review.
Cited by
-
How our hearts beat together: a study on physiological synchronization based on a self-paced joint motor task.Sci Rep. 2023 Jul 25;13(1):11987. doi: 10.1038/s41598-023-39083-9. Sci Rep. 2023. PMID: 37491507 Free PMC article.
-
Integrating Superior Hypogastric Plexus Block with Conventional Therapies: Advancements in Chronic Pelvic Pain Management.J Pharm Bioallied Sci. 2025 May;17(Suppl 1):S175-S178. doi: 10.4103/jpbs.jpbs_1813_24. Epub 2025 Apr 12. J Pharm Bioallied Sci. 2025. PMID: 40511122 Free PMC article. Review.
-
Impact of Endometriosis on Life-Course Potential: A Narrative Review.Int J Gen Med. 2021 Jan 7;14:9-25. doi: 10.2147/IJGM.S261139. eCollection 2021. Int J Gen Med. 2021. PMID: 33442286 Free PMC article. Review.
-
Myofascial Pain Syndrome in Women with Primary Dysmenorrhea: A Case-Control Study.Diagnostics (Basel). 2022 Nov 7;12(11):2723. doi: 10.3390/diagnostics12112723. Diagnostics (Basel). 2022. PMID: 36359567 Free PMC article.
-
Small Fiber Polyneuropathy May Be a Nexus Between Autonomic Nervous System Dysregulation and Pain in Interstitial Cystitis/Bladder Pain Syndrome.Front Pain Res (Lausanne). 2022 Jan 4;2:810809. doi: 10.3389/fpain.2021.810809. eCollection 2021. Front Pain Res (Lausanne). 2022. PMID: 35295485 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

