Harnessing neurovascular interaction to guide axon growth
- PMID: 30778117
- PMCID: PMC6379421
- DOI: 10.1038/s41598-019-38558-y
Harnessing neurovascular interaction to guide axon growth
Abstract
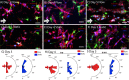
Regulating the intrinsic interactions between blood vessels and nerve cells has the potential to enhance repair and regeneration of the central nervous system. Here, we evaluate the efficacy of aligned microvessels to induce and control directional axon growth from neural progenitor cells in vitro and host axons in a rat spinal cord injury model. Interstitial fluid flow aligned microvessels generated from co-cultures of cerebral-derived endothelial cells and pericytes in a three-dimensional scaffold. The endothelial barrier function was evaluated by immunostaining for tight junction proteins and quantifying the permeability coefficient (~10-7 cm/s). Addition of neural progenitor cells to the co-culture resulted in the extension of Tuj-positive axons in the direction of the microvessels. To validate these findings in vivo, scaffolds were transplanted into an acute spinal cord hemisection injury with microvessels aligned with the rostral-caudal direction. At three weeks post-surgery, sagittal sections indicated close alignment between the host axons and the transplanted microvessels. Overall, this work demonstrates the efficacy of exploiting neurovascular interaction to direct axon growth in the injured spinal cord and the potential to use this strategy to facilitate central nervous system regeneration.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Lazarovici P, Marcinkiewicz C, Lelkes PI. Cross talk between the cardiovascular and nervous systems: neurotrophic effects of vascular endothelial growth factor (VEGF) and angiogenic effects of nerve growth factor (NGF)-implications in drug development. Curr Pharm Des. 2006;12:2609–2622. doi: 10.2174/138161206777698738. - DOI - PubMed
-
- Bar T. Patterns of vascularization in the developing cerebral cortex. Ciba Found Symp. 1983;100:20–36. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

