At the intersection of DNA damage and immune responses
- PMID: 30778174
- PMCID: PMC6438741
- DOI: 10.1038/s41577-019-0135-6
At the intersection of DNA damage and immune responses
Abstract
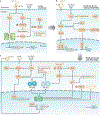
DNA damage occurs on exposure to genotoxic agents and during physiological DNA transactions. DNA double-strand breaks (DSBs) are particularly dangerous lesions that activate DNA damage response (DDR) kinases, leading to initiation of a canonical DDR (cDDR). This response includes activation of cell cycle checkpoints and engagement of pathways that repair the DNA DSBs to maintain genomic integrity. In adaptive immune cells, programmed DNA DSBs are generated at precise genomic locations during the assembly and diversification of lymphocyte antigen receptor genes. In innate immune cells, the production of genotoxic agents, such as reactive nitrogen molecules, in response to pathogens can also cause genomic DNA DSBs. These DSBs in adaptive and innate immune cells activate the cDDR. However, recent studies have demonstrated that they also activate non-canonical DDRs (ncDDRs) that regulate cell type-specific processes that are important for innate and adaptive immune responses. Here, we review these ncDDRs and discuss how they integrate with other signals during immune system development and function.
Figures






Similar articles
-
Function and Molecular Mechanism of the DNA Damage Response in Immunity and Cancer Immunotherapy.Front Immunol. 2021 Dec 14;12:797880. doi: 10.3389/fimmu.2021.797880. eCollection 2021. Front Immunol. 2021. PMID: 34970273 Free PMC article. Review.
-
A type I IFN-dependent DNA damage response regulates the genetic program and inflammasome activation in macrophages.Elife. 2017 Mar 31;6:e24655. doi: 10.7554/eLife.24655. Elife. 2017. PMID: 28362262 Free PMC article.
-
RAG-mediated DNA double-strand breaks activate a cell type-specific checkpoint to inhibit pre-B cell receptor signals.J Exp Med. 2016 Feb 8;213(2):209-23. doi: 10.1084/jem.20151048. Epub 2016 Feb 1. J Exp Med. 2016. PMID: 26834154 Free PMC article.
-
Regulation of the Cell-Intrinsic DNA Damage Response by the Innate Immune Machinery.Int J Mol Sci. 2021 Nov 25;22(23):12761. doi: 10.3390/ijms222312761. Int J Mol Sci. 2021. PMID: 34884568 Free PMC article. Review.
-
Eukaryotic DNA damage checkpoint activation in response to double-strand breaks.Cell Mol Life Sci. 2012 May;69(9):1447-73. doi: 10.1007/s00018-011-0875-3. Epub 2011 Nov 15. Cell Mol Life Sci. 2012. PMID: 22083606 Free PMC article. Review.
Cited by
-
Genome integrity and inflammation in the nervous system.DNA Repair (Amst). 2022 Nov;119:103406. doi: 10.1016/j.dnarep.2022.103406. Epub 2022 Sep 14. DNA Repair (Amst). 2022. PMID: 36148701 Free PMC article. Review.
-
The role of gut microbiota in intestinal disease: from an oxidative stress perspective.Front Microbiol. 2024 Feb 14;15:1328324. doi: 10.3389/fmicb.2024.1328324. eCollection 2024. Front Microbiol. 2024. PMID: 38419631 Free PMC article. Review.
-
DNA Damage Repair-Related Genes Signature for Immune Infiltration and Outcome in Cervical Cancer.Front Genet. 2022 Mar 3;13:733164. doi: 10.3389/fgene.2022.733164. eCollection 2022. Front Genet. 2022. PMID: 35309134 Free PMC article.
-
p53/NF-kB Balance in SARS-CoV-2 Infection: From OMICs, Genomics and Pharmacogenomics Insights to Tailored Therapeutic Perspectives (COVIDomics).Front Pharmacol. 2022 May 27;13:871583. doi: 10.3389/fphar.2022.871583. eCollection 2022. Front Pharmacol. 2022. PMID: 35721196 Free PMC article. Review.
-
Conventional DNA-Damaging Cancer Therapies and Emerging cGAS-STING Activation: A Review and Perspectives Regarding Immunotherapeutic Potential.Cancers (Basel). 2023 Aug 16;15(16):4127. doi: 10.3390/cancers15164127. Cancers (Basel). 2023. PMID: 37627155 Free PMC article. Review.
References
-
- Fugmann SD, Lee AI, Shockett PE, Villey IJ & Schatz DG The RAG proteins and V(D)J recombination: complexes, ends, and transposition. Annu. Rev. Immunol. 18, 495–527 (2000). - PubMed
-
- Chaudhuri J et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Adv. Immunol. 94, 157–214 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

