De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study
- PMID: 30778520
- PMCID: PMC6594455
- DOI: 10.1093/annonc/mdz055
De-escalated therapy for HR+/HER2+ breast cancer patients with Ki67 response after 2-week letrozole: results of the PerELISA neoadjuvant study
Abstract
Background: In human epidermal growth factor receptor 2 (HER2+) breast cancers, neoadjuvant trials of chemotherapy plus anti-HER2 treatment consistently showed lower pathologic complete response (pCR) rates in hormone receptor (HR) positive versus negative tumors. The PerELISA study was aimed to evaluate the efficacy of a de-escalated, chemotherapy-free neoadjuvant regimen in HR+/HER2+ breast cancer patients selected on the basis of Ki67 inhibition after 2-week letrozole.
Patients and methods: PerELISA is a phase II, multicentric study for postmenopausal patients with HR+/HER2+ operable breast cancer. Patients received 2-week letrozole, and then underwent re-biopsy for Ki67 evaluation. Patients classified as molecular responders (Ki67 relative reduction >20% from baseline) continued letrozole and started trastuzumab-pertuzumab for five cycles. Patients classified as molecular non-responders started weekly paclitaxel for 13 weeks combined with trastuzumab-pertuzumab. Primary aim was breast and axillary pCR. According to a two-stage Simon's design, to reject the null hypothesis, at least 8/43 pCR had to be documented.
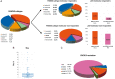
Results: Sixty-four patients were enrolled, 44 were classified as molecular responders. All these patients completed the assigned treatment with letrozole-trastuzumab-pertuzumab and underwent surgery. A pCR was observed in 9/44 cases (20.5%, 95% confidence interval 11.1% to 34.5%). Among molecular non-responders, 16/17 completed treatment and underwent surgery, with pCR observed in 81.3% of the cases. PAM50 intrinsic subtype was significantly associated with Ki67 response and pCR. Among molecular responders, the pCR rate was significantly higher in HER2-enriched than in other subtypes (45.5% versus 13.8%, P = 0.042).
Conclusions: The primary end point of the study was met, by reaching the pre-specified pCRs. In patients selected using Ki67 reduction after short-term letrozole exposure, a meaningful pCR rate can be achieved without chemotherapy. PAM50 intrinsic subtyping further refines our ability to identify a subset of patients for whom chemotherapy might be spared.
Eudract number: 2013-002662-40.
Clinicaltrials.gov identifier: NCT02411344.
Keywords: HER2-positive breast cancer; early breast cancer; neoadjuvant; pertuzumab; trastuzumab.
© The Author(s) 2019. Published by Oxford University Press on behalf of the European Society for Medical Oncology.
Figures
Comment in
-
ER+/HER2+ breast cancer: are we really de-escalating?Ann Oncol. 2019 Jun 1;30(6):875-877. doi: 10.1093/annonc/mdz130. Ann Oncol. 2019. PMID: 30993330 No abstract available.
Similar articles
-
HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: A correlative analysis from the PerELISA trial.EBioMedicine. 2022 Nov;85:104320. doi: 10.1016/j.ebiom.2022.104320. Epub 2022 Oct 29. EBioMedicine. 2022. PMID: 36374768 Free PMC article. Clinical Trial.
-
Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial.Lancet Oncol. 2020 Jan;21(1):33-43. doi: 10.1016/S1470-2045(19)30786-7. Epub 2019 Dec 11. Lancet Oncol. 2020. PMID: 31838010 Clinical Trial.
-
The role of immunohistochemistry in breast cancer patients treated with neoadjuvant chemotherapy: an old tool with an enduring prognostic value.Clin Breast Cancer. 2013 Apr;13(2):146-52. doi: 10.1016/j.clbc.2012.11.006. Epub 2013 Jan 11. Clin Breast Cancer. 2013. PMID: 23318089 Clinical Trial.
-
Dual HER2 Blockade in Neoadjuvant Treatment of HER2+ Breast Cancer: A Meta-Analysis and Review.Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820960721. doi: 10.1177/1533033820960721. Technol Cancer Res Treat. 2020. PMID: 32990165 Free PMC article. Review.
-
Molecular shifts in breast cancer following neoadjuvant chemotherapy: a prospective study and review of literature.Future Oncol. 2025 Apr;21(10):1209-1218. doi: 10.1080/14796694.2025.2475729. Epub 2025 Mar 24. Future Oncol. 2025. PMID: 40126169 Review.
Cited by
-
HER2DX genomic test in HER2-positive/hormone receptor-positive breast cancer treated with neoadjuvant trastuzumab and pertuzumab: A correlative analysis from the PerELISA trial.EBioMedicine. 2022 Nov;85:104320. doi: 10.1016/j.ebiom.2022.104320. Epub 2022 Oct 29. EBioMedicine. 2022. PMID: 36374768 Free PMC article. Clinical Trial.
-
Treatment strategies for hormone receptor-positive, human epidermal growth factor receptor 2-positive (HR+/HER2+) metastatic breast cancer: A review.Front Oncol. 2022 Dec 23;12:975463. doi: 10.3389/fonc.2022.975463. eCollection 2022. Front Oncol. 2022. PMID: 36620573 Free PMC article. Review.
-
A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed.Cancers (Basel). 2019 Nov 13;11(11):1782. doi: 10.3390/cancers11111782. Cancers (Basel). 2019. PMID: 31766180 Free PMC article. Review.
-
Therapeutic Strategies for the Management of Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Positive (HR+/HER2+) Breast Cancer: A Review of the Current Literature.Cancers (Basel). 2020 Nov 10;12(11):3317. doi: 10.3390/cancers12113317. Cancers (Basel). 2020. PMID: 33182657 Free PMC article. Review.
-
Molecular characterisation of the residual disease after neoadjuvant endocrine therapy in ER+/HER2- breast cancer uncovers biomarkers of tumour response.Transl Oncol. 2025 Jul;57:102407. doi: 10.1016/j.tranon.2025.102407. Epub 2025 May 10. Transl Oncol. 2025. PMID: 40349505 Free PMC article.
References
-
- Cortazar P, Zhang L, Untch M. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 2014; 384(9938): 164–172. - PubMed
-
- Guarneri V, Frassoldati A, Bottini A. et al. Preoperative chemotherapy plus trastuzumab, lapatinib, or both in human epidermal growth factor receptor 2-positive operable breast cancer: results of the randomized phase II CHER-LOB study. J Clin Oncol 2012; 30(16): 1989–1995. - PubMed
-
- Gianni L, Pienkowski T, Im Y-H. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13(1): 25–32. - PubMed
-
- Nahta R, O’Regan RM.. Therapeutic implications of estrogen receptor signaling in HER2-positive breast cancers. Breast Cancer Res Treat 2012; 135(1): 39–48. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

