Seroprotection at Different Levels of the Healthcare System After Routine Vaccination With Diphtheria-Tetanus-Pertussis whole cell-Hepatitis B-Haemophilus influenzae Type B in Lao People's Democratic Republic
- PMID: 30778522
- PMCID: PMC6880335
- DOI: 10.1093/cid/ciz143
Seroprotection at Different Levels of the Healthcare System After Routine Vaccination With Diphtheria-Tetanus-Pertussis whole cell-Hepatitis B-Haemophilus influenzae Type B in Lao People's Democratic Republic
Abstract
Background: The Lao People's Democratic Republic continues to sustain a considerable burden of vaccine-preventable diseases because of incomplete vaccine coverage and weak vaccine responses. We have assessed seroconversion after routine vaccination with the pentavalent vaccine to capture weaknesses of vaccine management at the different levels of the healthcare system.
Methods: A total of 1151 children (aged 8-28 months) with 3 documented doses of the pentavalent vaccine delivered at central hospitals in Vientiane and the provincial hospital, 3 district hospitals, and 10 health centers in Bolikhamxay province were enrolled. Sociodemographic information was collected with a standardized questionnaire. Serum samples were analyzed for antibodies against vaccine components, and bivariate and multivariable analyses were performed to identify risk factors for low vaccine responses.
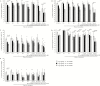
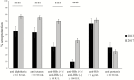
Results: Seroprotection rates at the provincial, district, and health center level were as high as in central hospitals, but seroprotection rates in areas covered by remote health centers were significantly lower. Protective levels also rapidly decreased with age at sampling. Seroprotection rates in Bolikhamxay against the different components reached 70%-77% and were up to 20% higher than in previous studies in the same region; 18.8% more children received the hepatitis B vaccine birth dose and the hepatitis B virus infection rate was 4 times lower.
Conclusions: Vaccine immunogenicity has dramatically improved in a central province, likely due to training and investment in the cold chain. Nevertheless, there remains a need to focus on the "last mile" in remote areas were most children are vaccinated through outreach activities.
Keywords: diphtheria; hepatitis B; immunogenicity; tetanus; vaccination.
© The Author(s) 2019. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Similar articles
-
Timeliness of immunisation with the pentavalent vaccine at different levels of the health care system in the Lao People's Democratic Republic: A cross-sectional study.PLoS One. 2020 Dec 8;15(12):e0242502. doi: 10.1371/journal.pone.0242502. eCollection 2020. PLoS One. 2020. PMID: 33290386 Free PMC article.
-
Low and disparate seroprotection after pentavalent childhood vaccination in the Lao People's Democratic Republic: a cross-sectional study.Clin Microbiol Infect. 2017 Mar;23(3):197-202. doi: 10.1016/j.cmi.2016.10.007. Epub 2016 Oct 15. Clin Microbiol Infect. 2017. PMID: 27756713
-
Safety and immunogenicity of a toddler dose following an infant series of a hexavalent diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b, hepatitis B vaccine administered concurrently or at separate visits with a heptavalent pneumococcal conjugate vaccine.Pediatr Infect Dis J. 2014 Jan;33(1):73-80. doi: 10.1097/01.inf.0000437806.76221.20. Pediatr Infect Dis J. 2014. PMID: 24346596 Clinical Trial.
-
Immunization of preterm infants with GSK's hexavalent combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type b conjugate vaccine: A review of safety and immunogenicity.Vaccine. 2018 Feb 8;36(7):986-996. doi: 10.1016/j.vaccine.2018.01.005. Epub 2018 Jan 12. Vaccine. 2018. PMID: 29336924 Review.
-
Immunogenicity of routinely used childhood vaccines when coadministered with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV).Pediatr Infect Dis J. 2009 Apr;28(4 Suppl):S97-S108. doi: 10.1097/INF.0b013e318199f61b. Pediatr Infect Dis J. 2009. PMID: 19325452 Review.
Cited by
-
Timeliness of immunisation with the pentavalent vaccine at different levels of the health care system in the Lao People's Democratic Republic: A cross-sectional study.PLoS One. 2020 Dec 8;15(12):e0242502. doi: 10.1371/journal.pone.0242502. eCollection 2020. PLoS One. 2020. PMID: 33290386 Free PMC article.
-
Iron Deficiency Anemia at Time of Vaccination Predicts Decreased Vaccine Response and Iron Supplementation at Time of Vaccination Increases Humoral Vaccine Response: A Birth Cohort Study and a Randomized Trial Follow-Up Study in Kenyan Infants.Front Immunol. 2020 Jul 13;11:1313. doi: 10.3389/fimmu.2020.01313. eCollection 2020. Front Immunol. 2020. PMID: 32754150 Free PMC article. Clinical Trial.
-
Factors associated with hepatitis B vaccination in Laos: findings from the multiple indicator cluster surveys in 2011/12 and 2017.Lancet Reg Health West Pac. 2024 Apr 15;46:101059. doi: 10.1016/j.lanwpc.2024.101059. eCollection 2024 May. Lancet Reg Health West Pac. 2024. PMID: 38645739 Free PMC article.
-
An age-stratified serosurvey against purified Salmonella enterica serovar Typhi antigens in the Lao People´s Democratic Republic.PLoS Negl Trop Dis. 2021 Dec 13;15(12):e0010017. doi: 10.1371/journal.pntd.0010017. eCollection 2021 Dec. PLoS Negl Trop Dis. 2021. PMID: 34898620 Free PMC article.
-
Susceptibility to Vaccine-Preventable Diseases in Four Districts of Xaysomboun Province, Lao People's Democratic Republic.Vaccines (Basel). 2022 Mar 17;10(3):463. doi: 10.3390/vaccines10030463. Vaccines (Basel). 2022. PMID: 35335095 Free PMC article.
References
-
- Evdokimov K, Sayasinh K, Nouanthong P, et al. . Low and disparate seroprotection after pentavalent childhood vaccination in the Lao People’s Democratic Republic: a cross-sectional study. Clin Microbiol Infect 2017; 23:197–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

