Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency
- PMID: 30778524
- PMCID: PMC6704391
- DOI: 10.1093/cvr/cvz045
Increased ketone body oxidation provides additional energy for the failing heart without improving cardiac efficiency
Abstract
Aims: The failing heart is energy-starved and inefficient due to perturbations in energy metabolism. Although ketone oxidation has been shown recently to increase in the failing heart, it remains unknown whether this improves cardiac energy production or efficiency. We therefore assessed cardiac metabolism in failing hearts and determined whether increasing ketone oxidation improves cardiac energy production and efficiency.
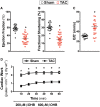
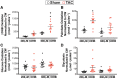
Methods and results: C57BL/6J mice underwent sham or transverse aortic constriction (TAC) surgery to induce pressure overload hypertrophy over 4-weeks. Isolated working hearts from these mice were perfused with radiolabelled β-hydroxybutyrate (βOHB), glucose, or palmitate to assess cardiac metabolism. Ejection fraction decreased by 45% in TAC mice. Failing hearts had decreased glucose oxidation while palmitate oxidation remained unchanged, resulting in a 35% decrease in energy production. Increasing βOHB levels from 0.2 to 0.6 mM increased ketone oxidation rates from 251 ± 24 to 834 ± 116 nmol·g dry wt-1 · min-1 in TAC hearts, rates which were significantly increased compared to sham hearts and occurred without decreasing glycolysis, glucose, or palmitate oxidation rates. Therefore, the contribution of ketones to energy production in TAC hearts increased to 18% and total energy production increased by 23%. Interestingly, glucose oxidation, in parallel with total ATP production, was also significantly upregulated in hearts upon increasing βOHB levels. However, while overall energy production increased, cardiac efficiency was not improved.
Conclusions: Increasing ketone oxidation rates in failing hearts increases overall energy production without compromising glucose or fatty acid metabolism, albeit without increasing cardiac efficiency.
Keywords: Beta-hydroxybutyrate; Cardiac Energy metabolism; Heart failure; Hypertrophy; Ketone body oxidation.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2019. For permissions, please email: journals.permissions@oup.com.
Figures




Comment in
-
Ketone body can be a fuel substrate for failing heart.Cardiovasc Res. 2019 Sep 1;115(11):1567-1569. doi: 10.1093/cvr/cvz104. Cardiovasc Res. 2019. PMID: 30989167 Free PMC article. No abstract available.
Similar articles
-
The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischaemic heart failure.Cardiovasc Res. 2024 Sep 2;120(10):1126-1137. doi: 10.1093/cvr/cvae092. Cardiovasc Res. 2024. PMID: 38691671 Free PMC article.
-
Ketones provide an extra source of fuel for the failing heart without impairing glucose oxidation.Metabolism. 2024 May;154:155818. doi: 10.1016/j.metabol.2024.155818. Epub 2024 Feb 17. Metabolism. 2024. PMID: 38369056
-
Preservation of Acyl Coenzyme A Attenuates Pathological and Metabolic Cardiac Remodeling Through Selective Lipid Trafficking.Circulation. 2019 Jun 11;139(24):2765-2777. doi: 10.1161/CIRCULATIONAHA.119.039610. Epub 2019 Mar 26. Circulation. 2019. PMID: 30909726 Free PMC article.
-
Advances in myocardial energy metabolism: metabolic remodelling in heart failure and beyond.Cardiovasc Res. 2024 Dec 14;120(16):1996-2016. doi: 10.1093/cvr/cvae231. Cardiovasc Res. 2024. PMID: 39453987 Free PMC article. Review.
-
Cardiac Energy Metabolism in Heart Failure.Circ Res. 2021 May 14;128(10):1487-1513. doi: 10.1161/CIRCRESAHA.121.318241. Epub 2021 May 13. Circ Res. 2021. PMID: 33983836 Free PMC article. Review.
Cited by
-
Metabolic and Signaling Roles of Ketone Bodies in Health and Disease.Annu Rev Nutr. 2021 Oct 11;41:49-77. doi: 10.1146/annurev-nutr-111120-111518. Annu Rev Nutr. 2021. PMID: 34633859 Free PMC article.
-
Novelties in the pharmacological approaches for chronic heart failure: new drugs and cardiovascular targets.Front Cardiovasc Med. 2023 Jun 2;10:1157472. doi: 10.3389/fcvm.2023.1157472. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37332581 Free PMC article. Review.
-
Impact of peroxisome proliferator-activated receptor-α on diabetic cardiomyopathy.Cardiovasc Diabetol. 2021 Jan 4;20(1):2. doi: 10.1186/s12933-020-01188-0. Cardiovasc Diabetol. 2021. PMID: 33397369 Free PMC article. Review.
-
Molecular Pathways in Diabetic Cardiomyopathy and the Role of Anti-hyperglycemic Drugs Beyond Their Glucose Lowering Effect.J Lipid Atheroscler. 2025 Jan;14(1):54-76. doi: 10.12997/jla.2025.14.1.54. Epub 2024 Nov 28. J Lipid Atheroscler. 2025. PMID: 39911956 Free PMC article. Review.
-
The ketogenic diet does not improve cardiac function and blunts glucose oxidation in ischaemic heart failure.Cardiovasc Res. 2024 Sep 2;120(10):1126-1137. doi: 10.1093/cvr/cvae092. Cardiovasc Res. 2024. PMID: 38691671 Free PMC article.
References
-
- Lopaschuk GD, Ussher JR, Folmes CDL, Jaswal JS, Stanley WC.. Myocardial fatty acid metabolism in health and disease. Physiol Rev 2010;90:207–258. - PubMed
-
- Lopaschuk GD, Ussher JR.. Evolving concepts of myocardial energy metabolism. Circ Res 2016;119:1173–1176. - PubMed
-
- De Jong KA, Lopaschuk GD.. Complex energy metabolic changes in heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Can J Cardiol 2017;33:860–871. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

