Monocytic C-C chemokine receptor 5 expression increases in in vitro intermittent hypoxia condition and in severe obstructive sleep apnea patients
- PMID: 30778913
- PMCID: PMC6867987
- DOI: 10.1007/s11325-019-01797-4
Monocytic C-C chemokine receptor 5 expression increases in in vitro intermittent hypoxia condition and in severe obstructive sleep apnea patients
Abstract
Purpose: Obstructive sleep apnea (OSA) patients have higher risk of cardiovascular disease. C-C chemokine receptor 5 (CCR5), as an important receptor for monocyte recruitment and the initiation of atherosclerosis, was studied under intermittent hypoxia and in OSA patients.
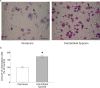
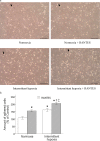
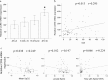
Methods: The expression and function of CCR5 regulated by intermittent hypoxia in monocytic THP-1 cells were investigated in an in vitro intermittent hypoxia culture system. The expression levels of protein and mRNA were analyzed by western blot and RT/real-time PCR analysis. Cell adhesion assay and transwell filter migration assay were carried out to investigate the adhesion and chemotaxis of monocytes. In addition, the mRNA expression of CCR5 in monocytes isolated from peripheral blood of 72 adults was analyzed.
Results: Intermittent hypoxia upregulated the expression of CCR5 in THP-1 cells and enhanced the adhesion and chemotaxis of monocytes to vascular endothelial cells mediated by RANTES. The CCR5 expression induced by intermittent hypoxia was inhibited by inhibitor for p42/44 MAPK. Besides, the expression of CCR5 in monocytes increased along the AHI value especially in severe OSA patients that was statistically significant compared with mild and moderate OSA groups.
Conclusions: This study demonstrated the increased monocytic CCR5 gene expression in patients with severe OSA. Intermittent hypoxia, the characteristic of OSA, induced monocytic CCR5 gene expression and the enhanced RANTES-mediated chemotaxis and adhesion through p42/44 MAPK signal pathways.
Keywords: Chemokine receptor; Chemotaxis; Intermittent hypoxia; Monocyte; Obstructive sleep apnea.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

