Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells
- PMID: 30778920
- PMCID: PMC6418078
- DOI: 10.1007/s13238-019-0608-1
Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells
Erratum in
-
Correction to: Modeling CADASIL vascular pathologies with patient-derived induced pluripotent stem cells.Protein Cell. 2024 May 7;15(5):393. doi: 10.1093/procel/pwad059. Protein Cell. 2024. PMID: 38113127 Free PMC article. No abstract available.
Abstract
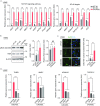
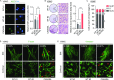
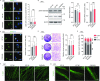
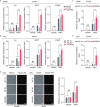
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is a rare hereditary cerebrovascular disease caused by a NOTCH3 mutation. However, the underlying cellular and molecular mechanisms remain unidentified. Here, we generated non-integrative induced pluripotent stem cells (iPSCs) from fibroblasts of a CADASIL patient harboring a heterozygous NOTCH3 mutation (c.3226C>T, p.R1076C). Vascular smooth muscle cells (VSMCs) differentiated from CADASIL-specific iPSCs showed gene expression changes associated with disease phenotypes, including activation of the NOTCH and NF-κB signaling pathway, cytoskeleton disorganization, and excessive cell proliferation. In comparison, these abnormalities were not observed in vascular endothelial cells (VECs) derived from the patient's iPSCs. Importantly, the abnormal upregulation of NF-κB target genes in CADASIL VSMCs was diminished by a NOTCH pathway inhibitor, providing a potential therapeutic strategy for CADASIL. Overall, using this iPSC-based disease model, our study identified clues for studying the pathogenic mechanisms of CADASIL and developing treatment strategies for this disease.
Keywords: CADASIL; NF-κB; NOTCH; iPSC; vascular smooth muscle.
Figures





References
-
- Agrinier N, Thilly N, Boivin JM, Dousset B, Alla F, Zannad F. Prognostic value of serum PIIINP, MMP1 and TIMP1 levels in hypertensive patients: a community-based prospective cohort study. Fundam Clin Pharmacol. 2013;27:572–580. - PubMed
-
- Andersson ER, Lendahl U. Therapeutic modulation of Notch signalling–are we there yet? Nat Rev Drug Discov. 2014;13:357–378. - PubMed
-
- Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

