Impaired Thermoregulatory Function during Dynamic Exercise in Multiple Sclerosis
- PMID: 30779715
- PMCID: PMC11742238
- DOI: 10.1249/MSS.0000000000001821
Impaired Thermoregulatory Function during Dynamic Exercise in Multiple Sclerosis
Abstract
Introduction: Impairments in sudomotor function during passive whole-body heating have been reported in multiple sclerosis (MS), a demyelinating disease of the CNS that disrupts autonomic function. However, the capability of the thermoregulatory system to control body temperature during exercise has never been assessed in MS. Thus, the aim of the present study was to test the hypothesis that thermoregulatory function is impaired in MS patients compared with healthy controls (CON) exercising at similar rates of metabolic heat production.
Methods: Sweating and skin blood flow responses were compared between 12 individuals diagnosed with relapsing-remitting MS (9 females, 3 males) and 12 sex-, age-, mass-, and BSA-matched CON during a single bout of cycling exercise (rate of metabolic heat production: ∼4.5 W·kg) for 60 min in a climate-controlled room (25°C, 30% RH).
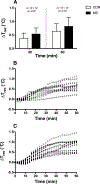
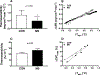
Results: Individuals with MS exhibited an attenuated increase in cumulative whole-body sweat loss after 30 min (MS, 72 ± 51 g; CON, 104 ± 37 g; P = 0.04) and 60 min (MS, 209 ± 94 g; CON, 285 ± 62 g; P = 0.02), as well as lower sweating thermosensitivity (MS, 0.49 ± 0.26 mg·cm·min·°C; CON, 0.86 ± 0.30 mg·cm·min·°C; P = 0.049). Despite evidence for thermoregulatory dysfunction, there were no differences between MS and CON in esophageal or rectal temperatures at 30- or 60-min time points (P > 0.05). Cutaneous vasculature responses were also not different in MS compared with CON (P > 0.05).
Conclusion: Taken together, MS blunts sweating responses during exercise while cutaneous vasculature responses are preserved. Altered mechanisms of body temperature regulation in persons with MS may lead to temporary worsening of disease symptoms and limit exercise tolerance under more thermally challenging conditions.
Conflict of interest statement
Conflicts of Interest:
No conflicts of interest, financial or otherwise, are declared by the authors. The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. The results and conclusions of the study do not constitute endorsement by the American College of Sports Medicine.
Figures


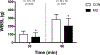

References
-
- Multiple Sclerosis: Hope Through Research | National Institute of Neurological Disorders and Stroke [Internet] [cited 2018 Aug 29]. Available from: https://www.ninds.nih.gov/disorders/patient-caregiver-education/hope-thr....
-
- Mcdonald WI. Pathophysiology in Multiple Sclerosis. Brain 1974;97(1):179–96. - PubMed
-
- Adamec I, Habek M. Autonomic dysfunction in multiple sclerosis. Clin Neurol Neurosurg 2013;115, Supplement 1:S73–8. - PubMed
-
- Flachenecker P, Wolf A, Krauser M, Hartung H-P, Reiners K. Cardiovascular autonomic dysfunction in multiple sclerosis: correlation with orthostatic intolerance. J Neurol 1999;246(7):578–86. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

