The impact of anxiety on chronic musculoskeletal pain and the role of astrocyte activation
- PMID: 30779717
- PMCID: PMC6407811
- DOI: 10.1097/j.pain.0000000000001445
The impact of anxiety on chronic musculoskeletal pain and the role of astrocyte activation
Abstract
Anxiety and depression are associated with increased pain responses in chronic pain states. The extent to which anxiety drives chronic pain, or vice versa, remains an important question that has implications for analgesic treatment strategies. Here, the effect of existing anxiety on future osteoarthritis (OA) pain was investigated, and potential mechanisms were studied in an animal model. Pressure pain detection thresholds, anxiety, and depression were assessed in people with (n = 130) or without (n = 100) painful knee OA. Separately, knee pain and anxiety scores were also measured twice over 12 months in 4730 individuals recruited from the general population. A preclinical investigation of a model of OA pain in normo-anxiety Sprague-Dawley (SD) and high-anxiety Wistar Kyoto (WKY) rats assessed underlying neurobiological mechanisms. Higher anxiety, independently from depression, was associated with significantly lower pressure pain detection thresholds at sites local to (P < 0.01) and distant from (P < 0.05) the painful knee in patients with OA. Separately, high anxiety scores predicted increased risk of knee pain onset in 3274 originally pain-free people over the 1-year period (odds ratio = 1.71; 95% confidence interval = 1.25-2.34, P < 0.00083). Similarly, WKY rats developed significantly lower ipsilateral and contralateral hind paw withdrawal thresholds in the monosodium iodoacetate model of OA pain, compared with SD rats (P = 0.0005). Linear regressions revealed that baseline anxiety-like behaviour was predictive of lowered paw withdrawal thresholds in WKY rats, mirroring the human data. This augmented pain phenotype was significantly associated with increased glial fibrillary acidic protein immunofluorescence in pain-associated brain regions, identifying supraspinal astrocyte activation as a significant mechanism underlying anxiety-augmented pain behaviour.
Conflict of interest statement
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
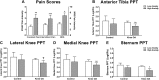
Figures



References
-
- Ackerman IN, Zomer E, Gilmartin-Thomas JFM, Liew D. Forecasting the future burden of opioids for osteoarthritis. Osteoarthritis Cartilage 2018;26:350–5. - PubMed
-
- Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, Graven-Nielsen T. Sensitization in patients with painful knee osteoarthritis. PAIN 2010;149:573–81. - PubMed
-
- Axford J, Butt A, Heron C, Hammond J, Morgan J, Alavi A, Bolton J, Bland M. Prevalence of anxiety and depression in osteoarthritis: use of the Hospital Anxiety and Depression Scale as a screening tool. Clin Rheumatol 2010;29:1277–83. - PubMed
-
- Barnett LA, Pritchard MG, Edwards JJ, Afolabi EK, Jordan KP, Healey EL, Finney AG, Chew-Graham CA, Mallen CD, Dziedzic KS. Relationship of anxiety with joint pain and its management: a population survey. Musculoskeletal Care 2018;16:353–62. - PubMed

