The relative toxicity of brodifacoum enantiomers
- PMID: 30779948
- PMCID: PMC6408973
- DOI: 10.1016/j.toxlet.2019.02.011
The relative toxicity of brodifacoum enantiomers
Abstract
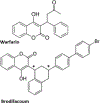
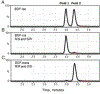
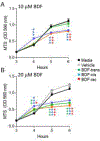
Brodifacoum (BDF) is a potent, long-acting anticoagulant rodenticide that can cause fatal poisoning in humans. The chemical structure of BDF includes 2 chiral carbons, resulting in 2 pairs of diastereomers, BDF-cis (R/S and S/R) and BDF-trans (R/R and S/S). However, the relative potency of these molecules is not known. The purpose of this study was to compare the in vitro and in vivo toxic effects of the 2 BDF diastereomer pairs. In adult Sprague-Dawley rats BDF-cis was significantly more toxic than BDF-trans (LD50 values of 219 versus 316 μg/kg, respectively) while racemic BDF had intermediate potency (266 μg/kg). In adult New Zealand white rabbits, BDF-cis had a longer half-life than BDF-trans which could contribute to its observed increased toxicity. Lastly, BDF-cis (10 μM), but not BDF-trans, damaged cultured SH-SY5Y human neuroblastoma cells by attenuating mitochondrial reductive capacity. Taken together, these data suggest that different toxic manifestations of BDF poisoning in mammals could be attributed, in part, to differences in relative enantiomer concentrations present in racemic formulations of this commercially-available toxicant.
Keywords: Anticoagulation; Brodifacoum; Diastereomer; Enantiomer; Superwarfarins.
Published by Elsevier B.V.
Conflict of interest statement
Declarations
DLF and IR are co-founders of EnSol Therapeutics, LLC. GW is co-founder of ResQ Pharmaceuticals, Inc. All other authors declare that they have no competing financial or non-financial interests.
Figures

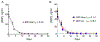
References
-
- Breckenridge A, Orme M, Wesseling H, Lewis RJ, Gibbons R, 1974. Pharmacokinetics and pharmacodynamics of the enantiomers of warfarin in man. Clinical pharmacology and therapeutics 15, 424–430. - PubMed
-
- Connors JM, 2018. Hemorrhagic Highs from Synthetic Cannabinoids - A New Epidemic. The New England journal of medicine 379, 1275–1277. - PubMed
-
- Damin-Pernik M, Espana B, Lefebvre S, Fourel I, Caruel H, Benoit E, Lattard V, 2017. Management of Rodent Populations by Anticoagulant Rodenticides: Toward Third-Generation Anticoagulant Rodenticides. Drug metabolism and disposition: the biological fate of chemicals 45, 160–165. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

