(-)-Oleocanthal Combined with Lapatinib Treatment Synergized against HER-2 Positive Breast Cancer In Vitro and In Vivo
- PMID: 30781364
- PMCID: PMC6412724
- DOI: 10.3390/nu11020412
(-)-Oleocanthal Combined with Lapatinib Treatment Synergized against HER-2 Positive Breast Cancer In Vitro and In Vivo
Abstract
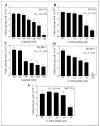
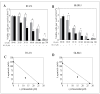
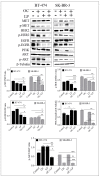
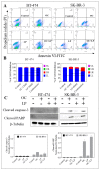
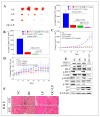
Dysregulation of epidermal growth factor receptor (EGFR)/human epidermal growth factor-2 (HER2) family is a hallmark of aggressive breast cancer. Small-molecule tyrosine kinase inhibitors are among the most effective cancer targeted treatments. (-)-Oleocanthal (OC) is a naturally occurring phenolic secoiridoid lead from extra-virgin olive oil with documented anti-cancer activities via targeting mesenchymal epithelial transition factor (c-Met). Dysregulation of c-Met promotes aggressiveness to breast cancer-targeted therapies. Lapatinib (LP) is an FDA-approved dual EGFR/HER2 inhibitor for HER2-amplified breast cancer. HER2-Positive tumor cells can escape targeted therapies like LP effects by overexpressing c-Met. Combined OC-LP treatment is hypothesized to be mechanistically synergistic against HER2-overexpressing breast cancer. Combined sub-effective treatments of OC-LP resulted in synergistic anti-proliferative effects against the HER2-positive BT-474 and SK-BR-3 breast cancer cell lines, compared to OC or LP monotherapy. Antibody array and Western blot analysis showed that combined OC-LP treatment significantly inhibited EGFR, HER2, and c-Met receptor activation, as well as multiple downstream signaling proteins, compared to individual OC or LP treatment. OC-LP Combination significantly inhibited invasion and migration of breast cancer cells through reduced activation of focal adhesion kinase (FAK) and paxillin. Combined treatment of OC-10 mg/kg with LP-12.5 mg/kg suppressed more than 90% of BT-474 tumor cells growth in a nude mouse xenograft model, compared to individual OC or LP treatment. Activated c-Met, EGFR, HER2, and protein kinase B (AKT) were significantly suppressed in combination-treated mice tumors, compared to OC or LP monotherapy. This study reveals the OC future potential as combination therapy to sensitize HER2-overexpressing breast cancers and significantly reduce required doses of targeted HER family therapeutics.
Keywords: (−)-Oleocanthal; HER2/neu; breast cancer; c-Met; combination; lapatinib.
Conflict of interest statement
K. El Sayed is a Medicinal Chemistry Chief Scientific Officer without compensation in the Shreveport, Louisiana-based Oleolive.
Figures

Similar articles
-
Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain.J Natl Cancer Inst. 2008 Aug 6;100(15):1092-103. doi: 10.1093/jnci/djn216. Epub 2008 Jul 29. J Natl Cancer Inst. 2008. PMID: 18664652 Free PMC article.
-
Epidermal growth factor receptor (HER1) tyrosine kinase inhibitor ZD1839 (Iressa) inhibits HER2/neu (erbB2)-overexpressing breast cancer cells in vitro and in vivo.Cancer Res. 2001 Dec 15;61(24):8887-95. Cancer Res. 2001. PMID: 11751413
-
Olive phenolics as c-Met inhibitors: (-)-Oleocanthal attenuates cell proliferation, invasiveness, and tumor growth in breast cancer models.PLoS One. 2014 May 21;9(5):e97622. doi: 10.1371/journal.pone.0097622. eCollection 2014. PLoS One. 2014. PMID: 24849787 Free PMC article.
-
Lapatinib.Recent Results Cancer Res. 2018;211:19-44. doi: 10.1007/978-3-319-91442-8_2. Recent Results Cancer Res. 2018. PMID: 30069757 Review.
-
Lapatinib: a small-molecule inhibitor of epidermal growth factor receptor and human epidermal growth factor receptor-2 tyrosine kinases used in the treatment of breast cancer.Clin Ther. 2009;31 Pt 2:2332-48. doi: 10.1016/j.clinthera.2009.11.029. Clin Ther. 2009. PMID: 20110044 Review.
Cited by
-
Oleocanthal, an Antioxidant Phenolic Compound in Extra Virgin Olive Oil (EVOO): A Comprehensive Systematic Review of Its Potential in Inflammation and Cancer.Antioxidants (Basel). 2023 Dec 14;12(12):2112. doi: 10.3390/antiox12122112. Antioxidants (Basel). 2023. PMID: 38136231 Free PMC article. Review.
-
An Appraisal of the Oleocanthal-Rich Extra Virgin Olive Oil (EVOO) and Its Potential Anticancer and Neuroprotective Properties.Int J Mol Sci. 2023 Dec 10;24(24):17323. doi: 10.3390/ijms242417323. Int J Mol Sci. 2023. PMID: 38139152 Free PMC article. Review.
-
(-)-Oleocanthal Nutraceuticals for Alzheimer's Disease Amyloid Pathology: Novel Oral Formulations, Therapeutic, and Molecular Insights in 5xFAD Transgenic Mice Model.Nutrients. 2021 May 18;13(5):1702. doi: 10.3390/nu13051702. Nutrients. 2021. PMID: 34069842 Free PMC article.
-
Optimization of Taste-Masked (-)-Oleocanthal Effervescent Formulation with Potent Breast Cancer Progression and Recurrence Suppressive Activities.Pharmaceutics. 2019 Oct 5;11(10):515. doi: 10.3390/pharmaceutics11100515. Pharmaceutics. 2019. PMID: 31590382 Free PMC article.
-
Safety Evaluations of Single Dose of the Olive Secoiridoid S-(-)-Oleocanthal in Swiss Albino Mice.Nutrients. 2020 Jan 25;12(2):314. doi: 10.3390/nu12020314. Nutrients. 2020. PMID: 31991771 Free PMC article.
References
-
- Konecny G.E., Pegram M.D., Venkatesan N., Finn R., Yang G., Rahmeh M., Untch M., Rusnak D.W., Spehar G., Mullin R.J., et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

