o-Vanillin Derived Schiff Bases and Their Organotin(IV) Compounds: Synthesis, Structural Characterisation, In-Silico Studies and Cytotoxicity
- PMID: 30781445
- PMCID: PMC6413231
- DOI: 10.3390/ijms20040854
o-Vanillin Derived Schiff Bases and Their Organotin(IV) Compounds: Synthesis, Structural Characterisation, In-Silico Studies and Cytotoxicity
Abstract
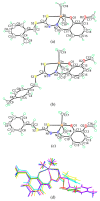
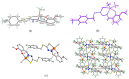
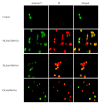
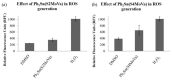
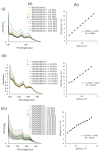
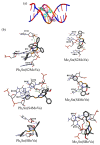
Six new organotin(IV) compounds of Schiff bases derived from S-R-dithiocarbazate [R = benzyl (B), 2- or 4-methylbenzyl (2M and 4M, respectively)] condensed with 2-hydroxy-3-methoxybenzaldehyde (oVa) were synthesised and characterised by elemental analysis, various spectroscopic techniques including infrared, UV-vis, multinuclear (¹H, 13C, 119Sn) NMR and mass spectrometry, and single crystal X-ray diffraction. The organotin(IV) compounds were synthesised from the reaction of Ph₂SnCl₂ or Me₂SnCl₂ with the Schiff bases (S2MoVaH/S4MoVaH/SBoVaH) to form a total of six new organotin(IV) compounds that had a general formula of [R₂Sn(L)] (where L = Schiff base; R = Ph or Me). The molecular geometries of Me₂Sn(S2MoVa), Me₂Sn(S4MoVa) and Me₂Sn(SBoVa) were established by X-ray crystallography and verified using density functional theory calculations. Interestingly, each experimental structure contained two independent but chemically similar molecules in the crystallographic asymmetric unit. The coordination geometry for each molecule was defined by thiolate-sulphur, phenoxide-oxygen and imine-nitrogen atoms derived from a dinegative, tridentate dithiocarbazate ligand with the remaining positions occupied by the methyl-carbon atoms of the organo groups. In each case, the resulting five-coordinate C₂NOS geometry was almost exactly intermediate between ideal trigonal-bipyramidal and square-pyramidal geometries. The cytotoxic activities of the Schiff bases and organotin(IV) compounds were investigated against EJ-28 and RT-112 (bladder), HT29 (colon), U87 and SJ-G2 (glioblastoma), MCF-7 (breast) A2780 (ovarian), H460 (lung), A431 (skin), DU145 (prostate), BE2-C (neuroblastoma) and MIA (pancreatic) cancer cell lines and one normal breast cell line (MCF-10A). Diphenyltin(IV) compounds exhibited greater potency than either the Schiff bases or the respective dimethyltin(IV) compounds. Mechanistic studies on the action of these compounds against bladder cancer cells revealed that they induced the production of reactive oxygen species (ROS). The bladder cancer cells were apoptotic after 24 h post-treatment with the diphenyltin(IV) compounds. The interactions of the organotin(IV) compounds with calf thymus DNA (CT-DNA) were experimentally explored using UV-vis absorption spectroscopy. This study revealed that the organotin(IV) compounds have strong DNA binding affinity, verified via molecular docking simulations, which suggests that these organotin(IV) compounds interact with DNA via groove-binding interactions.
Keywords: cytotoxic activity; dithiocarbazate; five-coordinate compounds; mechanistic studies; molecular docking; organotin(IV); single-crystal X-ray diffraction analysis; tridentate ONS Schiff bases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

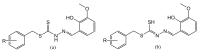


References
-
- Boseley S. [(accessed on 20 February 2018)]; Available online: https://www.theguardian.com/society/2014/feb/03/worldwide-cancer-cases-s....
-
- Global Cancer Observatory. [(accessed on 20 February 2018)]; Available online: http://gco.iarc.fr/
-
- Sirajuddin M., Ali S., Mckee V., Zaib S., Iqbal J. Organotin(IV) carboxylate derivatives as a new addition to anticancer and antileishmanial agents: Design, physicochemical characterization and interaction with Salmon sperm DNA. RSC Adv. 2014;4:57505–57521. doi: 10.1039/C4RA10487K. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

