A Novel MVA-Based HIV Vaccine Candidate (MVA-gp145-GPN) Co-Expressing Clade C Membrane-Bound Trimeric gp145 Env and Gag-Induced Virus-Like Particles (VLPs) Triggered Broad and Multifunctional HIV-1-Specific T Cell and Antibody Responses
- PMID: 30781504
- PMCID: PMC6410222
- DOI: 10.3390/v11020160
A Novel MVA-Based HIV Vaccine Candidate (MVA-gp145-GPN) Co-Expressing Clade C Membrane-Bound Trimeric gp145 Env and Gag-Induced Virus-Like Particles (VLPs) Triggered Broad and Multifunctional HIV-1-Specific T Cell and Antibody Responses
Abstract
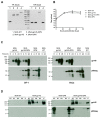
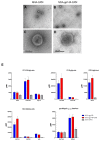
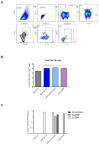
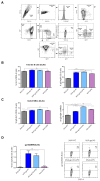
The development of an effective Human Immunodeficiency Virus (HIV) vaccine that is able to stimulate both the humoral and cellular HIV-1-specific immune responses remains a major priority challenge. In this study, we described the generation and preclinical evaluation of single and double modified vaccinia virus Ankara (MVA)-based candidates expressing the HIV-1 clade C membrane-bound gp145(ZM96) trimeric protein and/or the Gag(ZM96)-Pol-Nef(CN54) (GPN) polyprotein that was processed to form Gag-induced virus-like particles (VLPs). In vitro characterization of MVA recombinants revealed the stable integration of HIV-1 genes without affecting its replication capacity. In cells that were infected with Env-expressing viruses, the gp145 protein was inserted into the plasma membrane exposing critical epitopes that were recognized by broadly neutralizing antibodies (bNAbs), whereas Gag-induced VLPs were released from cells that were infected with GPN-expressing viruses. VLP particles as well as purified MVA virions contain Env and Gag visualized by immunoelectron microscopy and western-blot of fractions that were obtained after detergent treatments of purified virus particles. In BALB/c mice, homologous MVA-gp145-GPN prime/boost regimen induced broad and polyfunctional Env- and Gag-specific CD4 T cells and antigen-specific T follicular helper (Tfh) and Germinal Center (GC) B cells, which correlated with robust HIV-1-specific humoral responses. Overall, these results support the consideration of MVA-gp145-GPN vector as a potential vaccine candidate against HIV-1.
Keywords: CD4 T cells; Env-gp145; GC B cells; Gag-Pol-Nef; HIV-1; MVA vaccine; Tfh; VLPs; humoral responses; immunogenicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Virological and immunological characterization of novel NYVAC-based HIV/AIDS vaccine candidates expressing clade C trimeric soluble gp140(ZM96) and Gag(ZM96)-Pol-Nef(CN54) as virus-like particles.J Virol. 2015 Jan 15;89(2):970-88. doi: 10.1128/JVI.02469-14. Epub 2014 Oct 29. J Virol. 2015. PMID: 25355891 Free PMC article.
-
Prime-Boost Immunizations with DNA, Modified Vaccinia Virus Ankara, and Protein-Based Vaccines Elicit Robust HIV-1 Tier 2 Neutralizing Antibodies against the CAP256 Superinfecting Virus.J Virol. 2019 Apr 3;93(8):e02155-18. doi: 10.1128/JVI.02155-18. Print 2019 Apr 15. J Virol. 2019. PMID: 30760570 Free PMC article.
-
HIV/AIDS Vaccine Candidates Based on Replication-Competent Recombinant Poxvirus NYVAC-C-KC Expressing Trimeric gp140 and Gag-Derived Virus-Like Particles or Lacking the Viral Molecule B19 That Inhibits Type I Interferon Activate Relevant HIV-1-Specific B and T Cell Immune Functions in Nonhuman Primates.J Virol. 2017 Apr 13;91(9):e02182-16. doi: 10.1128/JVI.02182-16. Print 2017 May 1. J Virol. 2017. PMID: 28179536 Free PMC article.
-
Intrastructural help: improving the HIV-1 envelope antibody response induced by virus-like particle vaccines.Curr Opin HIV AIDS. 2017 May;12(3):272-277. doi: 10.1097/COH.0000000000000358. Curr Opin HIV AIDS. 2017. PMID: 28422791 Review.
-
Justification for the inclusion of Gag in HIV vaccine candidates.Expert Rev Vaccines. 2016 May;15(5):585-98. doi: 10.1586/14760584.2016.1129904. Epub 2015 Dec 28. Expert Rev Vaccines. 2016. PMID: 26645951 Review.
Cited by
-
High throughput analysis of B cell dynamics and neutralizing antibody development during immunization with a novel clade C HIV-1 envelope.PLoS Pathog. 2023 Oct 25;19(10):e1011717. doi: 10.1371/journal.ppat.1011717. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37878666 Free PMC article.
-
An MVA Vector Expressing HIV-1 Envelope under the Control of a Potent Vaccinia Virus Promoter as a Promising Strategy in HIV/AIDS Vaccine Design.Vaccines (Basel). 2019 Dec 6;7(4):208. doi: 10.3390/vaccines7040208. Vaccines (Basel). 2019. PMID: 31817622 Free PMC article.
-
An engineered HIV-1 Gag-based VLP displaying high antigen density induces strong antibody-dependent functional immune responses.NPJ Vaccines. 2023 Apr 6;8(1):51. doi: 10.1038/s41541-023-00648-4. NPJ Vaccines. 2023. PMID: 37024469 Free PMC article.
-
Nucleic Acid Vaccines Encoding Proteins and Virus-like Particles for HIV Prevention.Vaccines (Basel). 2024 Mar 12;12(3):298. doi: 10.3390/vaccines12030298. Vaccines (Basel). 2024. PMID: 38543932 Free PMC article. Review.
-
The Envelope-Based Fusion Antigen GP120C14K Forming Hexamer-Like Structures Triggers T Cell and Neutralizing Antibody Responses Against HIV-1.Front Immunol. 2019 Dec 4;10:2793. doi: 10.3389/fimmu.2019.02793. eCollection 2019. Front Immunol. 2019. PMID: 31867001 Free PMC article.
References
-
- Garcia F., de Quiros J.C., Gomez C.E., Perdiguero B., Najera J.L., Jimenez V., Garcia-Arriaza J., Guardo A.C., Perez I., Diaz-Brito V., et al. Safety and immunogenicity of a modified pox vector-based HIV/AIDS vaccine candidate expressing Env, Gag, Pol and Nef proteins of HIV-1 subtype b (MVA-B) in healthy HIV-1-uninfected volunteers: A phase I clinical trial (RISVAC02) Vaccine. 2011;29:8309–8316. doi: 10.1016/j.vaccine.2011.08.098. - DOI - PubMed
-
- Gomez C.E., Najera J.L., Perdiguero B., Garcia-Arriaza J., Sorzano C.O., Jimenez V., Gonzalez-Sanz R., Jimenez J.L., Munoz-Fernandez M.A., de Quiros J.C., et al. The HIV/AIDS vaccine candidate MVA-B administered as a single immunogen in humans triggers robust, polyfunctional, and selective effector memory T cell responses to HIV-1 antigens. J. Virol. 2011;85:11468–11478. doi: 10.1128/JVI.05165-11. - DOI - PMC - PubMed
-
- Guardo A.C., Gomez C.E., Diaz-Brito V., Pich J., Arnaiz J.A., Perdiguero B., Garcia-Arriaza J., Gonzalez N., Sorzano C.O.S., Jimenez L., et al. Correction: Safety and vaccine-induced HIV-1 immune responses in healthy volunteers following a late MVA-B boost 4 years after the last immunization. PLoS ONE. 2018;13:e0195915. doi: 10.1371/journal.pone.0195915. - DOI - PMC - PubMed
-
- Joseph S., Quinn K., Greenwood A., Cope A.V., McKay P.F., Hayes P.J., Kopycinski J.T., Gilmour J., Miller A.N., Geldmacher C., et al. A comparative phase I study of combination, homologous subtype-C DNA, MVA, and Env gp140 protein/adjuvant HIV vaccines in two immunization regimes. Front. Immunol. 2017;8:149. doi: 10.3389/fimmu.2017.00149. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

